In a mass spectrograph, the masses of ions are determined from their deflections in a magnetic field.
Question:
In a mass spectrograph, the masses of ions are determined from their deflections in a magnetic field. Suppose that singly charged ions of chlorine are shot perpendicularly into a magnetic field B = 0.15 T with a speed of 5.0 × 104 m/s. (The speed could be measured by use of a velocity selector.) Chlorine has two major isotopes, of masses 34.97 u and 36.97 u. What would be the radii of the circular paths described by the two isotopes in the magnetic field? (See Fig. 45-1.)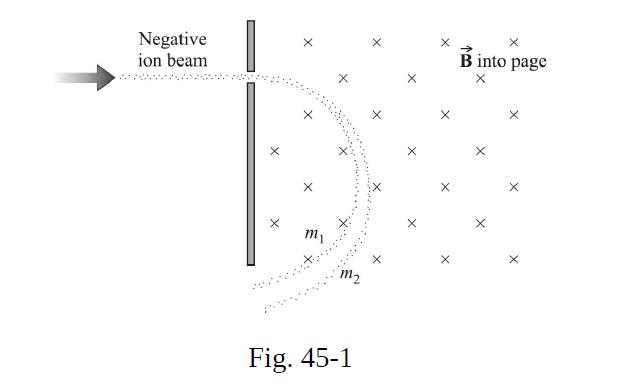
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





