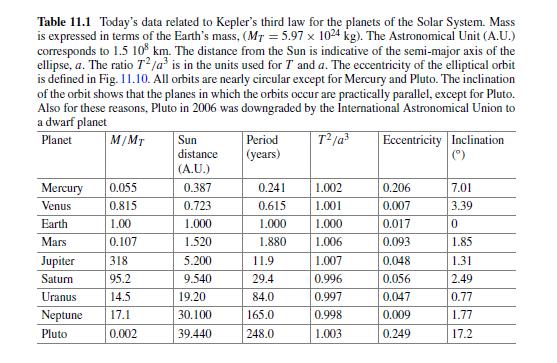
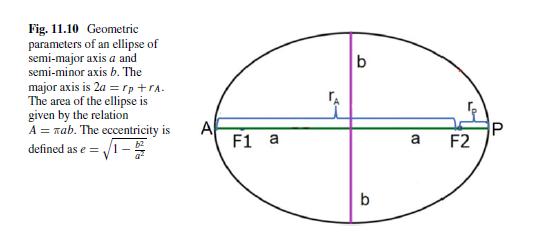
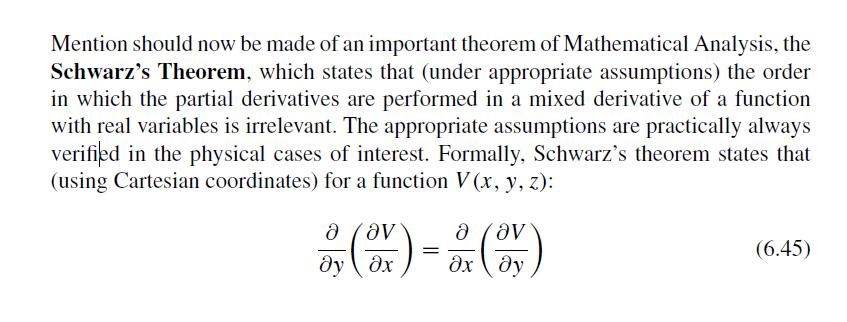
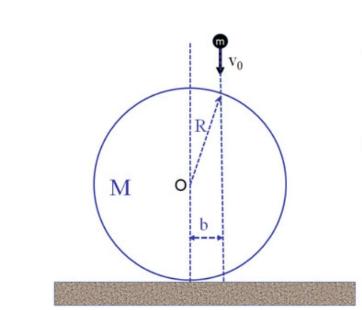
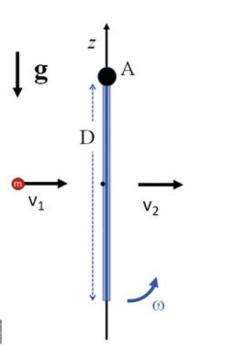
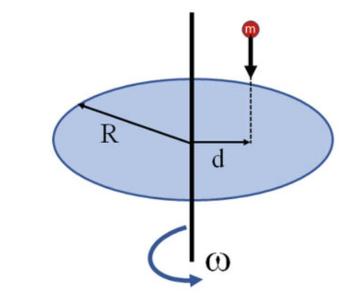
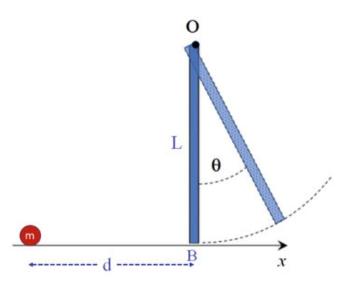
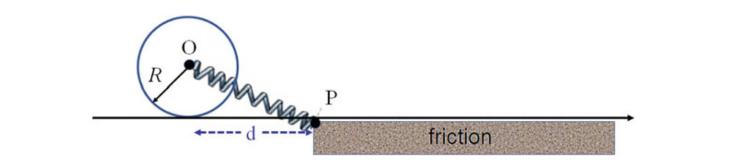
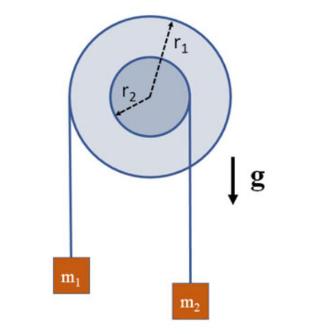
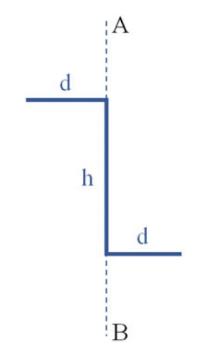
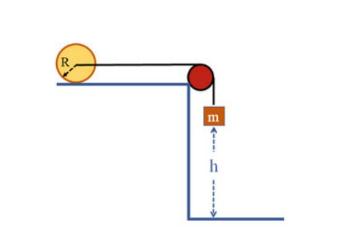
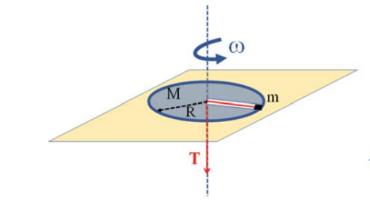
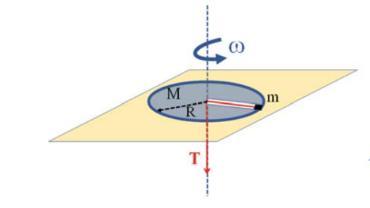
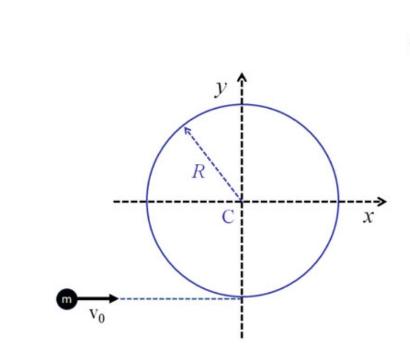
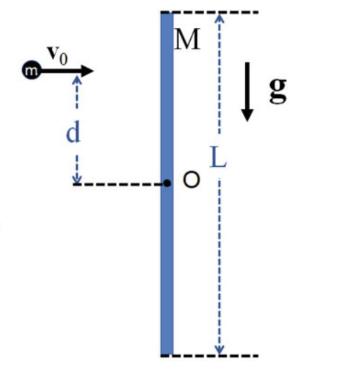
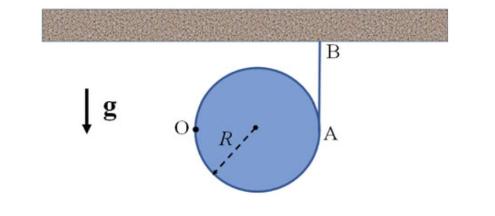
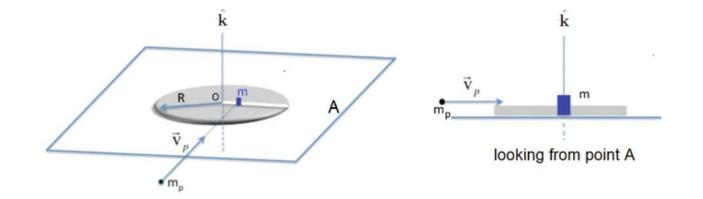
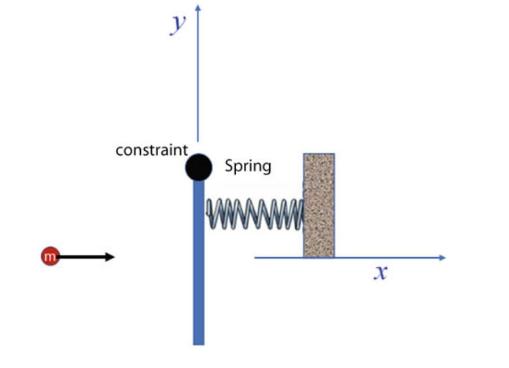
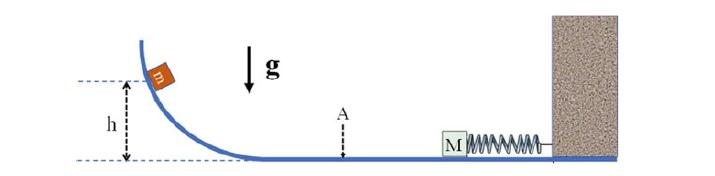
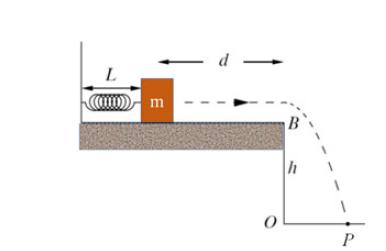
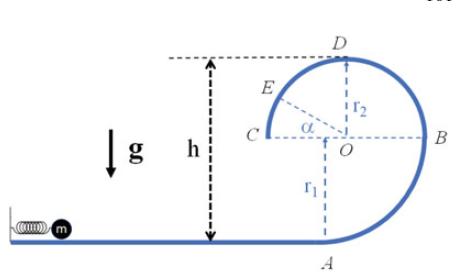
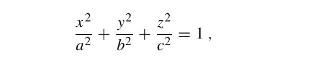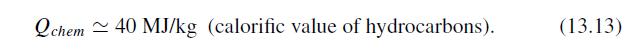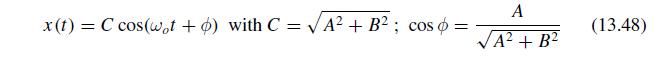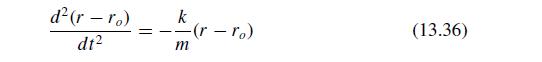The Fundamentals Of Newtonian Mechanics 1st Edition Maurizio Spurio - Solutions
Discover the comprehensive guide to "The Fundamentals Of Newtonian Mechanics 1st Edition" by Maurizio Spurio, featuring expertly crafted solutions and solved problems. Access the complete solution manual with step-by-step answers, providing a valuable resource for understanding the key concepts of this textbook. Our online platform offers a free download of the answers key and solutions PDF, making your study experience seamless and efficient. Delve into detailed chapter solutions and utilize the instructor manual for enhanced learning. Whether you're tackling a test bank or seeking questions and answers, our resources provide the perfect support for mastering Newtonian mechanics.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()