Question: A distillation unit consists of a partial reboiler, a bubble-cap column, and a total condenser. The overall plate efficiency is 65%. The feed is a
A distillation unit consists of a partial reboiler, a bubble-cap column, and a total condenser. The overall plate efficiency is 65%. The feed is a bubble-point liquid of 50 mol% benzene in toluene, which is fed to the optimal plate. The column is to produce a distillate containing 95 mol% benzene and a bottoms of 95 mol% toluene. Calculate for an operating pressure of 1 atm the:
(a) Minimum reflux ratio (L / D)min;
(b) Minimum number of actual plates;
(c) Number of actual plates needed for a reflux ratio (L/D) of 50% more than the minimum;
(d) Kg/h of distillate and bottoms, if the feed is 907.3 kg/h; and
(e) Saturated steam at 273.7 kPa required in kg/h for the reboiler using the enthalpy data below and any assumptions necessary.
(f) Make a rigorous enthalpy balance on the reboiler, using the enthalpy data below and assuming ideal solutions. Enthalpies are in Btu/lbmol at reboiler temperature:
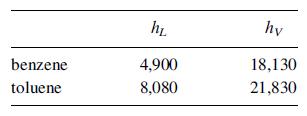
Vapor–liquid equilibrium data are given in Exercise 7.13.
Data From Exercise 7.13
A column at 101 kPa is to separate 30 kg/h of a bubble-point solution of benzene and toluene containing 0.6 mass-fraction toluene into an overhead product of 0.97 mass-fraction benzene and a bottoms product of 0.98 mass-fraction toluene at a reflux ratio of 3.5. The feed is sent to the optimal tray, and the reflux is at saturation temperature. Determine the:
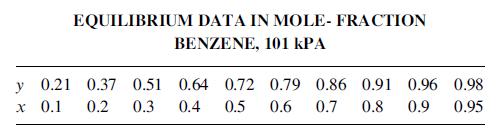
benzene toluene hL 4,900 8,080 hy 18,130 21,830
Step by Step Solution
3.41 Rating (154 Votes )
There are 3 Steps involved in it
a Minimum reflux ratio L Dmin 109 b Minimum number of actual plates 8 c Number ... View full answer

Get step-by-step solutions from verified subject matter experts


