A vapor mixture of equal volumes NH 3 and N 2 is contacted at 20 C
Question:
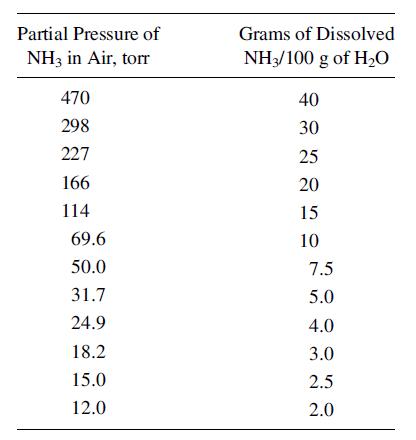
A vapor mixture of equal volumes NH3 and N2 is contacted at 20οC and 1 atm (760 torr) with water to absorb some of the NH3. If 14 m3 of this mixture is contacted with 10 m3 of water, calculate the % of ammonia in the gas that is absorbed. Both T and P are maintained constant. The partial pressure of NH3 over water at 20οC is:
Transcribed Image Text:
Partial Pressure of NH3 in Air, torr 470 298 227 166 114 69.6 50.0 31.7 24.9 18.2 15.0 12.0 Grams of Dissolved NH3/100 g of H₂O 40 30 25 20 15 10 7.5 5.0 4.0 3.0 2.5 2.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
The of ammonia in the gas that is absorbed 14 m3 of gas 1410 m3 of wat...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper
Question Posted:
Students also viewed these Life Sciences questions
-
The volume of chlorine at 20 C and 1 atm of pressure is 150 L. What volume will it occupy at 60 C and 2770 mmHg of pressure?
-
What volume of O2 at 20 C and 1 atm of pressure can be obtained from 100 g of KClO3?
-
A vapor mixture of n-butane (B) and n-hexane (H) contains 50.0 mole% butane at 120C and 1.0 atm, A stream of this mixture flowing at a rate of 150.0 L/s is cooled and compressed, causing some but not...
-
Data-2-Go manufactures and sells flash drives. The company produces only when it receives orders and, therefore, has no inventories. The following information is available for the current month:...
-
An investigator wants to take an unequal-probability sample of 10 of the 25 psus in the population listed below and in file exercise0602.dat, and wishes to sample units with replacement. a. Adapt the...
-
What services are provided by investment banks? Do they compete with commercial banks?
-
Learned how to find probabilities of discrete events by using the probability rules of Chapter 3. LO8
-
In your audit of Aviary Industries for calendar year 2016, you found a number of matters that you believe represent possible adjustments to the company's books. These matters are described below....
-
Assume you are working with the accounting department in your organization to make a decision regarding a capital investment you feel is needed to improve productivity in your department. The...
-
The following data were obtained from a series of Charpy impact tests performed on four steels, each having a different manganese content. Plot the data and determine (a) The transition temperature...
-
Fifty mol% propylene in propane is separated with silica gel. The products are to be 90 mol% propylene and 75 mol% propane. If 1,000 lb of silica gel/lbmol of feed gas is used, can the desired...
-
A colored substance (B) is removed from a mineral oil by adsorption with clay particles at 25C. The original oil has a color index of 200 units/100 kg oil, while the decolorized oil must have an...
-
Adding computerized medical images to a database promises to provide great resources for physicians.However, there are other methods of obtaining such information, so the issue of efficiency of...
-
# III: Worksheet 3 1. A 20 kg mass is allowed to accelerate down a frictionless 15 ramp. 20 kg 15 a. Draw a force diagram for the block. b. Determine the value of the x-component of the force of...
-
3.Baker Corporation has provided the following production and average cost data for two levels of monthly production volume. The company produces a single product Production Volume: 1,000 units:...
-
Suppose that you own the only company in the market to produce a certain product, and therefore you can determine the market price P dollars for each unit. Due to government regulations, the price of...
-
describes how the blast pressure front can bounce off solid, immovable obstacles and be redirected in another direction in a linear angle to the angle of the obstacle hat was struck
-
As the accounting clerk, you are tasked by the CFO to determine the cost of goods sold of Del Mundo Company for the year ended December 31, 2020. During Operating cost data annd inventory account...
-
Determine whether the given sequence is arithmetic, geometric, or neither. If the sequence is arithmetic, find the common difference; if it is geometric, find the common ratio. If the sequence is...
-
A new car sold for $31,000. If the vehicle loses 15% of its value each year, how much will it be worth after 10 years?
-
Many viruses, including WNV, cold viruses, and flu viruses, reproduce in the host for a short period of time before being destroyed by the hosts immune system. This production of new virus particles...
-
In the acquired immune response, two types of lymphocytes, B cells and T cells, are the key players. a. Compare and contrast the antigen receptors of B and T cells. b. B and T cells can only...
-
Each tassel produces 25 million pollen grains. One acre of a cornfield may contain 20,000 to 30,000 corn plants, producing up to 68 kg (approximately 150 pounds) of pollen in a single growing season....
-
Bass Accounting Services expects its accountants to work a total of 26,000 direct labor hours per year. The company's estimated total indirect costs are $ 260,000. The company uses direct labor hours...
-
The Balance Sheet has accounts where the accountant must make estimates. Some situations in which estimates affect amounts reported in the balance sheet include: Allowance for doubtful accounts....
-
Alado fis istirmerfs Tat likifond 205L [ridont inip lanod whadtinion? hingend is antan Qultit foer avdeed Divdasit errem yodichiders Etexlpoges Getmare nelp

Study smarter with the SolutionInn App


