Question: Fifty mol% propylene in propane is separated with silica gel. The products are to be 90 mol% propylene and 75 mol% propane. If 1,000 lb
Fifty mol% propylene in propane is separated with silica gel. The products are to be 90 mol% propylene and 75 mol% propane. If 1,000 lb of silica gel/lbmol of feed gas is used, can the desired separation be made in one stage? If not, what separation can be achieved? Use Figure 4.29.
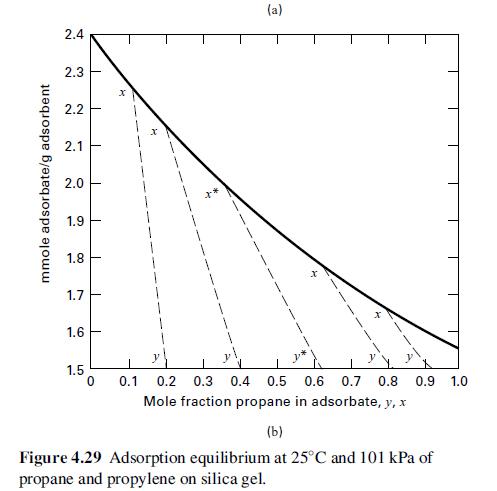
mmole adsorbate/g adsorbent 2.4 2.3 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 T T 1 1 1 I 1 0 X (a) 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 Mole fraction propane in adsorbate, y, x (b) Figure 4.29 Adsorption equilibrium at 25C and 101 kPa of propane and propylene on silica gel.
Step by Step Solution
3.41 Rating (157 Votes )
There are 3 Steps involved in it
To determine if the desired separation of 50 mol propylene in propane into 9... View full answer

Get step-by-step solutions from verified subject matter experts


