Question: A gas containing 50 mol% propylene in propane is to be separated with silica gel having the equilibrium properties shown in Figure. The final products
A gas containing 50 mol% propylene in propane is to be separated with silica gel having the equilibrium properties shown in Figure. The final products are to be 90 mol% propylene and 75 mol% propane. If 1,000 lb of silica gel/lbmol of feed gas or less is used, can the desired separation be made in one equilibrium stage? If not, what separation can beachieved?
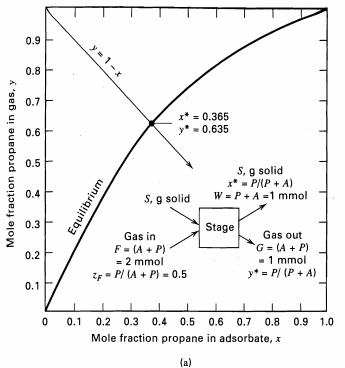
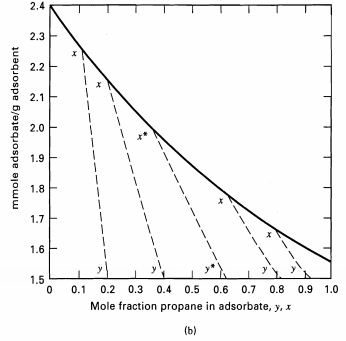
0.9 0.8 0.7 x* = 0.365 - 0.635 0.6 0.5 S, g solid x* - P/(P + A) W = P+ A =1 mmol 0.4 S, g solid 0.3 Stage Gas out Gas in G = (A + P) -1 mmol y* = P/ (P + A) F = (A + P) = 2 mmol 0.2 ZF - P/ (A + P) - 0.5 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 0.1 Mole fraction propane in adsorbate, x (a) y= 1-x Mole fraction propane in gas, y Equilibrium
Step by Step Solution
3.46 Rating (169 Votes )
There are 3 Steps involved in it
With a basis of 1 lbmole of feed gas let y and x be the mole fractions of P in the equilibrium vapor ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (157).docx
120 KBs Word File


