A vaporliquid mixture at 250F and 500 psia contains N 2 , H 2 S, CO 2
Question:
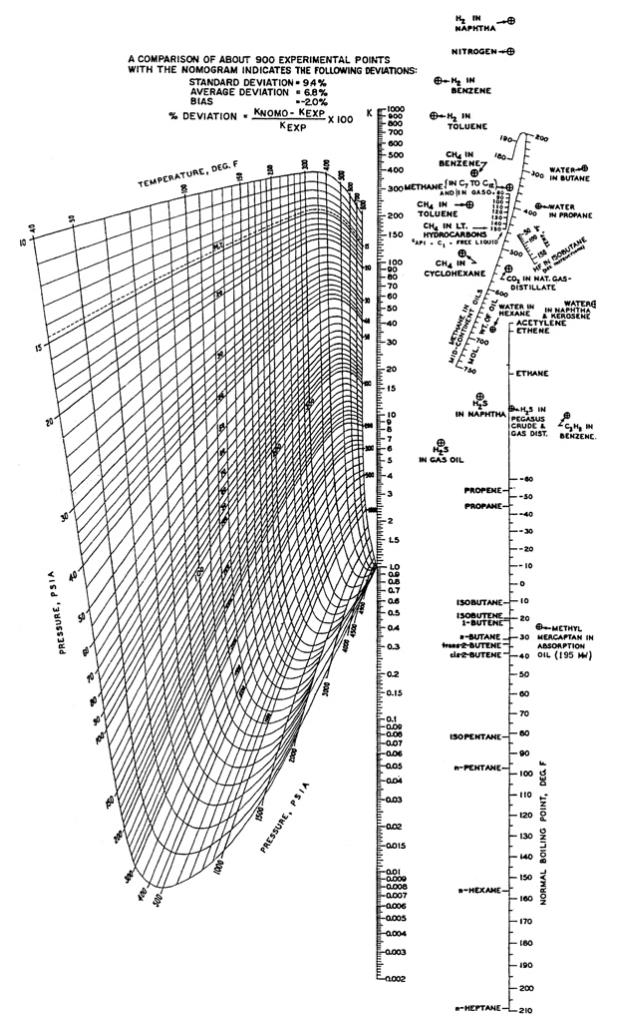
A vapor–liquid mixture at 250ºF and 500 psia contains N2, H2S, CO2, and all the normal paraffins from methane to heptane. Use Figure 2.4 to estimate the K-value of each component. Which components will be present to a greater extent in the equilibrium vapor?
Transcribed Image Text:
2 Cr PRESSURE, PSIA 3 A COMPARISON OF ABOUT 900 EXPERIMENTAL POINTS WITH THE NOMOGRAM INDICATES THE FOLLOWING DEVIATIONS 500. -005 STANDARDEVIATION 6.8% **20% BIAS % DEVIATION KNOMO-EXP X 100 Do PRESSURE, PSIA MID- NI ********** 'דידי 0 1 8 2 8 8 8 8 8 8 8 8 NORMAL BOILING POINT, DEG F 2 8 8 8
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
Answer The Kvalues of the components are given in the table be...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper
Question Posted:
Students also viewed these Life Sciences questions
-
A 250 L rigid tank contains methane at 500 K, 1500 kPa. It is now cooled down to 300 K. Find the mass of methane and the heat transfer using a) ideal gas and b) the methane tables.
-
A 250 L rigid tank contains methane at 500 K, 1500 kPa. It is now cooled down to 300 K. Find the mass of methane and the heat transfer using a) ideal gas and b) the methane tables.
-
A water storage tank contains liquid and vapor in equilibrium at 110C. The distance from the bottom of the tank to the liquid level is 8 m. What is the absolute pressure at the bottom of the tank?
-
Five different laboratories participated in an interlaboratory study involving determinations of the iron level in water samples. The results below are replicate determinations of Fe in ppm for...
-
What are the strengths and weaknesses of e-distribution?
-
Why can't the expected growth rate exceed the investor's required return in the constant growth model?
-
33. How do partners measure the amount they have at risk in the partnership?
-
Depreciation for Partial PeriodsSL, Act, SYD, and DDB on January 1, 2008, a machine was purchased for $90,000. The machine has an estimated salvage value of $6,000 and an estimated useful life of 5...
-
At the end of its first year of operations, Shapiro's Consulting Services reported net income of $27,000. They also had account balances of: Cash, $16,000; Office Supplies, $3,200; Equipment, $24,000...
-
From the information in BE5.4, prepare the journal entries to record these transactions on McLeena Companys books under a perpetual inventory system. Data From BE 5.4 Prepare the journal entries to...
-
Benzene can break the ethanol/water azeotrope to produce nearly pure ethanol. Wilson constants for the ethanol (1)/benzene (2) system at 45 C are L 12 = 0.124 and L 21 = 0.523. Use these with...
-
One thousand kmol/h of rich gas at 70F with 25% C 1 , 15% C 2 , 25% C 3 , 20% nC 4 , and 15% nC 5 by moles is to be absorbed by 500 kmol/h of nC 10 at 90F in an absorber at 4 atm. Calculate by the...
-
Define basic and diluted EPS.
-
When in 1920 the Chia brothers opened their first shop in Bangkok selling seeds for farmers, they did not know that they were on the way to launching the development of one of the most successful...
-
In Exercises 49-52, sketch a plane. Then sketch the described situation. Three noncollinear points that lie in the plane
-
In Exercises 49-52, sketch a plane. Then sketch the described situation. A plane perpendicular to the given plane
-
Trace the polygon and point P on paper. Then draw a rotation of the polygon the given number of degrees about P. 150 F P G
-
Trace the polygon and point P on paper. Then draw a rotation of the polygon the given number of degrees about P. 30 B C
-
What was Alphabet Inc.s (Google) accounting effective tax rate for 2017? What items caused the companys accounting effective tax rate to differ from the hypothetical tax rate of 35 percent? What was...
-
Solve each equation. x 3 - 6x 2 = -8x
-
How can RNAi gene silencing be used to determine the function of genes?
-
How do insertional mutagenesis approaches differ from other reverse genetic approaches?
-
Insertional mutagenesis is a powerful tool in both plants and animals. However, when performing large-scale insertional mutagenesis, what major advantage do plants have over animals?
-
Practice Problem 1 The stockholders equity accounts of Bramble Corp. on January 1, 2017, were as follows. Preferred Stock (6%, $100 par noncumulative, 4,400 shares authorized) $264,000 Common Stock...
-
JVCU Which of the following is considered cash for financial reporting purposes? 1 JVCU Which of the following is considered cash for financial reporting purposes? 1
-
Required information The Foundational 15 [LO8-2, LO8-3, LO8-4, LO8-5, LO8-7, LO8-9, L08-10) (The following information applies to the questions displayed below.) Morganton Company makes one product...

Study smarter with the SolutionInn App


