Derive general expressions for (a) The partition coefficient, K o D , in an organic (1) aqueous(2)
Question:
Derive general expressions for
(a) The partition coefficient, KoD, in an organic (1) aqueous–(2) LLE system for an un-ionized (neutral) form of a weak base given by
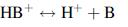
and
(b) The distribution coefficient between organic and aqueous fluid phases in terms of pH and pKa.
Transcribed Image Text:
HB+ → H+ + B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (9 reviews)
a The partition coefficient is defined as KoDfracC1eqC2eq where C1eq and C2eq are the equilibrium co...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper
Question Posted:
Students also viewed these Life Sciences questions
-
The base B is too weak to titrate in aqueous solution. (a) Which solvent, pyridine or acetic acid, would be more suitable for the titration of B with HClO4? Why? (b) Which solvent would be more...
-
A 0.150 M aqueous solution of the weak organic acid HA was prepared from the pure compound, and three 50.0-mL aliquots were transferred to 100.0-mL volumetric flasks. Solution 1 was diluted to 100.0...
-
Derive an expression for the relationship between pKa and pKb for a conjugate acidbase pair.
-
Do you consider the Internet message boards that Anjali wishes to use to be public or private? How would you justify your answer? Anjali, was in her final year of study for an undergraduate business...
-
In an SRS, each possible subset of n units has probability of being chosen as the sample; in this chapter, we showed that this definition implies that each unit has probability n/N of appearing in...
-
Exploring Ancient Mysteries Develop an essay which adequately addresses the topic you have chosen. Tutankhamen died young, at approximately eighteen (18) years of age. However, his cause of death has...
-
Because training is costly, when times are tough, some hospitality operations reduce their training budgets in order to save costs. A. True B. False
-
How many types of bankruptcy and these types explained in Chapters?
-
Hi there; this is just a list of terms that I could just google, but I wanted a sort of different explanation / definition if that's okay. That's all! (They're just Business Law terms) 1. Assignment...
-
Nancy Nanny opened a child-care facility on January 1, Year 1. Use the Chart of Accounts below to complete the requirements on the following pages for Nancy Nanny Child Care. NANCY NANNY CHILD...
-
Cocochips is a manufacturer of vegan snacks and treats, including a unique brand of granola and a distinct brand of coconut-flavoured chips and snacks. The company began its operations in 20X8, and...
-
Actinomycin D present at 260 mg/L in fermentation broth is to be extracted into butyl acetate. At pH 3.5, the distribution constant is 57. How many stages are required to recover 99% of the...
-
32 Value of Right Show that the value of a right can be written as Value of a right = P RO P X = ( P RO P S )/ (N+1) where PRO, PS and PX stand for the rights-on price, the subscription price and...
-
Root cause analysis with fish bone diagram and Forecast analysis for the case study "Agarwal Automobiles: Fuel station forecasting and inventory management" with peer reviewed journal references.
-
Suppose that MPI_COMM WORLD consists of the eight processes 0, 1, 2, 3, 4, 5, 6, and 7, and suppose the following code is executed: int sum = my_sum; int iLevel = 0; MPI Status status; for (int...
-
Measuring and monitoring It is the SMT's view that the reduction in accident frequency rate alone clearly indicates that the slips and trips campaign was a success discuss possible limitations of...
-
Identify some of the repercussions of high staff turnover at Eswatini Electricity Company ( EEC ) , especially on critical and skilled employees occupying key positions. Further, differentiate...
-
1. Make sure your report server is setup correctly. 2. Deploy all 10 reports and Shared Data Source. 3. Take a snapshot of each report (Parameters Visible) and paste them in 1 MS Word Document. The...
-
Find x so that 2x, 3x + 2 and 5x + 3 are consecutive terms of an arithmetic sequence.
-
Does log 81 (2401) = log 3 (7)? Verify the claim algebraically.
-
All five characters are present in which taxon? (A) (B) (C) (G) (H)
-
The gecko is a reptile with an amazing ability to climb smooth surfaces, including glass. Recent discoveries indicate that geckos stick to smooth surfaces via van der Waals interactions between...
-
Much of what we know about cellular function depends on experiments utilizing specific cells and specific parts (e.g., organelles) of cells. What techniques do scientists commonly use to isolate...
-
exercise 4-7 (Algo) Effects of transactions on income statement LO P2
-
Compute the value of ordinary bonds under the following circumstances assuming that the coupon rate is 0.06:(either the correct formula(s) or the correct key strokes must be shown here to receive...
-
A tax-exempt municipal bond has a yield to maturity of 3.92%. An investor, who has a marginal tax rate of 40.00%, would prefer and an otherwise identical taxable corporate bond if it had a yield to...

Study smarter with the SolutionInn App


