Assume that bcc iron at 1800 K has a fraction of vacant Fe sites of 0.0001, an
Question:
Assume that bcc iron at 1800 K has a fraction of vacant Fe sites of 0.0001, an Fe atom vibration frequency
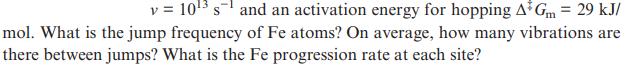
Transcribed Image Text:
v = 10 s and an activation energy for hopping A Gm = 29 kJ/ mol. What is the jump frequency of Fe atoms? On average, how many vibrations are there between jumps? What is the Fe progression rate at each site?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Equation 39 Fe atom has N 8 nearest neighbors The jump frequenc...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
Calculate the self-diffusion coefficient of Fe in Problem 3.22, given the unit-cell parameter a = 2.87 . Problem 3.22 Assume that bcc iron at 1800 K has a fraction of vacant Fe sites of 0.0001, an Fe...
-
A BCC iron structure is to be manufactured that will allow no more than 50 g of hydrogen to be lost per year through each square centimeter of the iron at 400 C. If the concentration of hydrogen at...
-
Iron and vanadium both have the BCC crystal structure and V forms a substitutional solid solution in Fe for concentrations up to approximately 20 wt% V at room temperature. Determine the...
-
The compound bow in the previous problem requires 152 J of work to draw the string back to x = 0.540 m, at which point the archer need only exert 250 N of force to hold the string in place. (a)...
-
Determine the polar moment of inertia and the radius of gyration of the isosceles triangle shown with respect to point O.
-
Imperial Brass Ltd. wanted to computerize all of its systems. Jacob Electric Systems Ltd. presented Imperial with a proposal that met Imperials needs. In August, Imperial accepted the proposal, along...
-
(p. 46). Certified Public Accountants (CPAs) were surveyed on their firms likelihood of reporting sustainability policies (measured as a probability between 0 and 1). The CPAs were divided into four...
-
Carol Gorden was a good friend of yours in high school and is from your home town. While you chose to major in accounting when you both went away to college, she majored in marketing and management....
-
Suppose that XYZ Corp. will generate free-cash-flows (FCF) of $400 at the end of the year. XYZ has a cost of equity capital of 12%, a cost of debt capital of 5%, a market value debt-to-equity ratio...
-
Given the diffusivity DC = 10 10 m 2 /s for interstitial carbon in bcc iron at 800 C of a= = 103 S-) 2.87 , estimate the activation energy A Gm for hopping of the C atoms (v= via interstitial sites.
-
Calculate the jump frequency of interstitial carbon in bcc iron at 800 C, assuming a vibration frequency v = 10 s and an activation energy for hopping A*Gm 62 kJ/mol. On average, every x-th carbon...
-
What conclusions can you draw from the results of the four previous exercises?
-
Waverly Company Ltd. currently produces 8,000 units per year of SB 200 (snowboard), which is a component of the company's major products. SB 200 has the following unit cots Direct materials - $35.50...
-
Norton Ltd manufactures a single product, which is sold for $150 per unit. The standard variable costs per unit of the product are: Direct material 4 kilos at $8 per kilo Direct labour 5 hours at $10...
-
QUESTION 4 Murni Selasih Bhd is considering investing in a project that will generate higher returns Currently, the company has two projects with forecasted outcomes under consideration. The possible...
-
ABC plans to sell 60,000 units of product 751 in June, and each of these units requires five sq. ft. of raw material. Additional data is as follows: Product Raw No. 751 Material Actual June 1 11,200...
-
Case: Tom has felt anxious and constantly on edge over the past 3 years. He has few social contacts because of his nervous symptoms. He is married with 3 children and worries about if he is a good...
-
Health Canada claims that a pharmaceutical company makes cold caplets that contain amounts of acetaminophen with a mean different from the 650 mg amount indicated on the label. Assume that a...
-
Using (1) or (2), find L(f) if f(t) if equals: t cos 4t
-
Compare the following three isomeric dienes: (a) Which compound will liberate the least heat upon hydrogenation with 2 mol of hydrogen gas? Why? (b) Which compound will liberate the most heat upon...
-
Identify the most stable compound:
-
Draw an energy diagram showing the relative energy levels of the MOs for 1,3,5,7- octatetraene and identify the HOMO and LUMO for both the ground state and the excited state.
-
Which of the following are elements of a bootstrappable business model? Indicate ALL that apply. Large up-front capital investment Recurring revenue stream Long sales cycles Word of mouth advertising
-
Hooligan Adventure Supply produces and sells various outdoor equipment. The Molding and Assembly production departments are supported by the Personnel and Maintenance departments. Personnel costs are...
-
Kelley Enterprises In October 1989, Pat Kelley.wus in his office, preparing the 1990 budget and contemplating the recent races of his business. Orders had been plentiful Lately that he though that...

Study smarter with the SolutionInn App


