Compare the following three isomeric dienes: (a) Which compound will liberate the least heat upon hydrogenation with
Question:
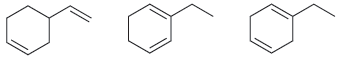
(a) Which compound will liberate the least heat upon hydrogenation with 2 mol of hydrogen gas? Why?
(b) Which compound will liberate the most heat upon hydrogenation with 2 mol of hydrogen gas? Why?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (9 reviews)
a The conjugated diene will liberate the least heat because it is the most stabl...View the full answer

Answered By

Anum Naz
Lecturer and researcher with 10+ years of experience teaching courses in both undergraduate and postgraduate levels. Supervised 17 BA theses, 07 MA theses, and 1 Ph.D. dissertations. Edited and co-authored 2 monographs on contemporary trends in political thought. Published over articles in peer-reviewed journals.
4.80+
11+ Reviews
52+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
What spectroscopic method could you use to distinguish among the following three isomeric acids? Tell what characteristic features you would expect for eachacid. CH3(CH213CO2H (CH/2CHCH-cO2H...
-
Solid NaI is slowly added to a solution that is 0.010 M in Cu1 and 0.010 M in Ag1. (a) Which compound will begin to precipitate first? (b) Calculate [Ag1] when CuI just begins to precipitate. (c)...
-
Which compound will undergo an electrophilic aromatic substitution reaction more rapidly, benzene or hexadeuteriobenzene? or D- H.
-
= Adobe Reader Touch Type here to search Active Research: Toyota's Hybrid Offer One of the most successful hybrid cars (cars that run on both battery and gasoline) is the Prius by Toyota. Visit...
-
What are three examples of data models? Which one is the most popular type?
-
In Exercises use the Special Integration Formulas (Theorem 8.2) to find the indefinite integral. Data from in Theorem 8.2 THEOREM 8.2 Special Integration Formulas (a > 0) 1. 1. S - 1 du = = 1/(= 2....
-
Define assimilation and its importance to understanding how people deal with change. AppendixLO1
-
When viscous dissipation is included, Equation 8.48 (multiplied by pcp) becomes This problem explores the importance of viscous dissipation. The conditions under consideration are laminar, fully...
-
Henrie's Drapery Service is investigating the purchase of a new machine for cleaning and blocking drapes. The machine would cost $102,990, including freight and installation. Henrie's has estimated...
-
Middleboro Township plans to order supplies every quarter of the year. It expects to receive the supplies in the quarter after they are ordered. It expects to use them the quarter after that and pay...
-
Identify each of the following quotes as being an example of either: the coordination problem, the invisible hand, creative destruction, or the incentive problem. a. "If you compare a list of todays...
-
True or False: Households sell finished products to businesses.
-
A spherical snowball is melting. Find the approximate change in volume if the radius decreases from 3 cm to 2.8 cm.
-
! Required information [The following information applies to the questions displayed below.] Littleton Books has the following transactions during May. May 2 Purchases books on account from Readers...
-
4) Consider the table to the right that shows the number of free samples () and number of protein shakes sold (y). a)Complete the table [3 marks] b) Find the equation of the line of best fit X x y 8...
-
Determine the mean number of credit cards based on the raw data. (b) Determine the standard deviation number of credit cards based on the raw data. (c) Determine a probability distribution for the...
-
B Harry is a county Department of Social Services worker whose clients consist primarily of poor, female-headed families receiving public assistance. During one of his meetings with Dora, a single...
-
1 A, Weakly coupled carts (20 points) m m2 Figure 1: A system of two masses and three springs. A symmetric two degree of freedom system consists of two identical rigid masses m = m = m pictured in...
-
Dynamic Express Inc. prepared the summary shown below regarding its investments on December 31, 2023, its year-end. Prepare the appropriate entry on December 31, 2023, to record the fair value...
-
Quality Chicken grows and processes chickens. Each chicken is disassembled into five main parts. Information pertaining to production in July 2012 is: Joint cost of production in July 2012 was $50. A...
-
What product would you expect to obtain from addition of Cl2 to 1, 2-dimcthyl- cyclohexane? Show the stereochemistry of the product.
-
Addition of HC1 to 1, 2-dimethylcyclohexene yields a mixture of two products. Show the stereochemistry of each, and explain why a mixture is formed.
-
What product would you expect from the reaction of cyclopentane with NBS and water? Show the stereo chemistry.
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


