What spectroscopic method could you use to distinguish among the following three isomeric acids? Tell what characteristic
Question:
What spectroscopic method could you use to distinguish among the following three isomeric acids? Tell what characteristic features you would expect for eachacid.
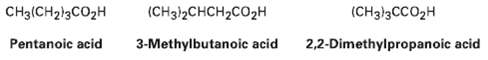
Transcribed Image Text:
CH3(CH213CO2H (CHз/2CHCH-cO2H (CHз])3ССO2H Pentanoic acid 3-Methylbutanoic acid 2,2-Dimethylpropanoic acid
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 41% (12 reviews)
Either 3C NMR or H NMR can be used to distinguish ...View the full answer

Answered By

Ashish Jaiswal
I have completed B.Sc in mathematics and Master in Computer Science.
4.90+
20+ Reviews
39+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Distinguish among the following three risks: risk-free interest rate, business risk, and information risk. Which one or ones does the auditor reduce by per-forming an audit?
-
Distinguish among the following types of operational audits: functional, organizational, and special assignment. State an example of each for a not-for-profit hospital.
-
Distinguish among the following concepts: (a) Difference between book value and the value implied by the purchase price. (b) Excess of implied value over fair value. (c) Excess of fair value over...
-
Write a paper on the brothers karamazov, fyodor dostoyevsky creates a conversation between two brothers: the cynical ivan, and the devoutly religious and earnest alyosha. In their dialogues...
-
With so much business being conducted over the Internet, including email, why is it important to understand cross-cultural differences in values?
-
If q = 2.90, MS Residual = 12.27, and n = 8, what is HSD?
-
Think about a sporting organisation you are familiar with. Do you think the approach taken by Clive Woodward would be successful in that organisation? Give reasons for your answer.
-
In 2010, Elbert Corporation had net cash provided by operating activities of $531,000; net cash used by investing activities of $963,000; and net cash provided by financing activities of $585,000. At...
-
Why would an investor want to beat the market versus hold the market? Discuss the strategies for each and their dependence on an investors information and trading skills.
-
Icebreaker Company (a U.S.-based company) sells parts to a foreign customer on December 1, 2020, with payment of 16,000 dinars to be received on March 1, 2021. Icebreaker enters into a forward...
-
Propose a structure for a compound C 6 H 12 O 2 that dissolves in dilute NaOH and shows the following 1 H NMR spectrum: 1.08 (9 H, singlet), 2.2 (2 H, singlet), and 11.2 (1 H, singlet).
-
How would you use NMR (either 13C or 1H) to distinguish between the following pairs of isomers? (a) C and 2 (b) 2CH2CH2CO2H CH3CH(CO2H)2 and (c) CH3CH2CH2C02H and H-CH2CH2CH and -2 (d)...
-
According to MarketWatch, the mean net worth of all individuals in the United States in 2016 was $692,100, while the median net worth was $97,300. (a) Which measure do you believe better describes...
-
7. A psychiatrist is testing a new ADHD Medication, which seems to have the potentially harmful side effect of increasing the heart rate. For a sample of 50 clinical study participants whose pulse...
-
Determine the type of engagement that your colleague completed for the client. Justify the selected engagement type for the client. Assess the purpose of each financial statement for the client's...
-
Mills Corporation acquired as a long-term investment $235 million of 8% bonds, dated July 1, on July 1, 2024. Company management has classified the bonds as an available-for-sale investment. The...
-
A force of 28 pounds acts on the pipe wrench shown in the figure below. 18 in. 30 (a) Find the magnitude of the moment about O by evaluating ||OA x F||. (0 0 180) Use a graphing utility to graph the...
-
Module 1 1. There has been a rise in cases of measles in RI. The RI Health Department is wondering if the rate of MMR vaccinations has declined since the start of the COVID-19 pandemic. The...
-
Compare and contrast the initial AllRoad process, shown in Figure 2-1, with the version shown in Figure CE18-4. (Treat the parallelograms in Figure 2-1 as databases.) a. Summarize the problems that...
-
The following data are supplied for the common stocks of Nikola Corporation, Tesla, Inc. and General Motors: Nikola Corp (NKLA) Tesla Inc. (TSLA) Close Price ($) Close Price ($) 67.53 30.00 40.81...
-
Rhodium has a density of 12.41 g/cm 3 and crystallizes with the face-centered cubic unit cell. Calculate the radius of a rhodium atom.
-
Consider two 2p orbitals, one on each of two different atoms, oriented side-to-side, as in Figure Pl.46. Imagine bringing these nuclei together so that overlap occurs as shown in the figure. This...
-
Consider two 2p orbitals, one on each of two different atoms, oriented side-to-side, as in Figure Pl.46. Imagine bringing these nuclei together so that overlap occurs as shown in the figure. This...
-
Consider two 2p orbitals, one on each of two different atoms, oriented side-to-side, as in Figure Pl.46. Imagine bringing these nuclei together so that overlap occurs as shown in the figure. This...
-
On consolidated financial statements, where does the parents equity in the net income of the subsidiary account appear? A. On the consolidated income statement, as a revenue B. On the consolidated...
-
Which of the following is not one of the elements of the balanced scorecard? a.cost system b.strategic initiatives c.performance targets d.strategy maps
-
Yield to Maturity and Call with Semiannual Payments Shares Remaining After Recapitalization Dye Trucking raised $75 million in new debt and used this to buy back stock. After the recap, Dye's stock...

Study smarter with the SolutionInn App


