How would you use NMR (either 13C or 1H) to distinguish between the following pairs of isomers?
Question:
How would you use NMR (either 13C or 1H) to distinguish between the following pairs of isomers?
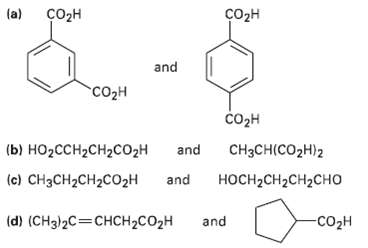
Transcribed Image Text:
(a) созн Cоон and со2н соон (b) НО2СCH2CH2CO2H CH3CH(CO2H)2 and (c) CH3CH2CH2C02H and носH-CH2CH2CHО and -со2н (d) (CH3)2C=CHCH2CO2H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
In all of these pairs different numbers of peaks occur in the spectra of each iso...View the full answer

Answered By

Anoop V
I have five years of experience in teaching and I have National Eligibility in teaching (UGC-NET) .
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How could you distinguish between the following pairs of isomers by 1H NMR spectroscopy? a. CH3CCl3 and CH2ClCHCl2 b. CH3CH2CH2OH and (CH3)2CHOH c d. 0 C-OCH and H C OCH2CH CH2-CH=O and C CH
-
How would you use 1 H NMR spectroscopy to distinguish between the following compounds? (a) (b) (c) (d) (e) (f) . . . CI
-
Distinguish between the following pairs of terms: a. Debt securities classified as held to maturity versus available for sale b. Equity securities classified as trading securities versus available...
-
Write a critical review paper on the topic of financial management in the broad sense.
-
A wheelchair user is conducting a job search to land a middle-management position. Given that so many companies are attempting to build a more culturally diverse group of managers, should this job...
-
If MS Columns = 66.54 and MS Within = 78.88, what is F Columns ?
-
What do you think were the most important changes made which allowed England to be successful under Clive Woodward? Why?
-
PEI Potatoes processes potatoes into potato cuts at its highly automated plant. For many years, it processed potatoes for only the retail consumer market where it had a superb reputation for quality....
-
During Tax Year 2023, Mary spent $20,000 on new solar panels for her residence located in the United States. Her tax liability is $2,736. What is Mary's Residential Clean Energy Credit carryforward...
-
Julio and Milania are owners of Falcons Corporation, an S corporation. They each own 50 percent of Falcons Corporation. In year 1, Julio and Milani received distributions of $20,000 and $10,000,...
-
What spectroscopic method could you use to distinguish among the following three isomeric acids? Tell what characteristic features you would expect for eachacid. CH3(CH213CO2H (CH/2CHCH-cO2H...
-
Compound A, C 4 H 8 O 3 , has infrared absorptions at 1710 and 2500 to 3100 cm ?1 and has the 1 H NMR spectrum shown. Propose a structure for A. 11.18 TMS 10 O ppm 8. 6. 3. Chemical shift (6)...
-
What advantages are gained by separating fixed expenses from variable expenses? It has been said that the statement fluctuating overheads are those which vary with output is an unjustifiable...
-
Determine the magnitude of the magnetic flux through the south-facing window of a house in British Columbia, where Earth's B field has a magnitude of 5.8 x 10-5T and the direction of B field is 72...
-
A wedge with an inclination of angle rests next to a wall. A block of mass m is sliding down the plane, as shown. There is no friction between the wedge and the block or between the wedge and the...
-
Conner Leonard worked for Purges Manufacturing for 32 years. Along with four other men, he helped to start the company that designed and built products sold around the world. Purges Manufacturing...
-
Reconsider the collision between two objects diagrammed below where two objects move on a frictionless surface. Before collision After collision Experiment 1 A, 1 B A B Draw complete and properly...
-
3. Now the bomb arrives. Please catch fx,y(x, y) = = cx cx - dy, where 0 < x < 1, 0 y x. 13 a) Please find coefficients c, d such that cd= 8 b) Please find fx(x) and fy (y). Are X and Y independent?...
-
Suppose you are starting a business that is similar to AllRoad. a. Should you start with business processes first or information systems first? Defend your answer. b. Most likely, you will obtain...
-
The baseball player A hits the ball from a height of 3.36 ft with an initial velocity of 34.8 ft/s. 0.14 seconds after the ball is hit, player B who is standing 15 ft away from home plate begins to...
-
Molybdenum crystallizes with the body-centered unit cell. The radius of a molybdenum atom is 136 pm. Calculate the edge length of the unit cell and the density of molybdenum.
-
Draw a Lewis structure for acetonitrile, C2H3N, assuming that all bonding obeys the octet rule, and that no atom bears a formal charge. Acetonitrile contains a carbon-nitrogen triple bond.
-
Compute the formal charges on atom of the following structure. what is the charge on the entire structure? (a) :0: :0 P- :O:
-
Compute the formal charges on atom of the following structure. what is the charge on the entire structure? (a) :0: :0 P- :O:
-
Milano Pizza is a small neighborhood pizzeria that has a small area for in-store dining as well as offering take-out and free home delivery services. The pizzerias owner has determined that the shop...
-
Which of the following statement regarding a post-closing trial balance is not true
-
What are the benefits and potential risks factors for undertaking derivative strategies compared to cash transactions

Study smarter with the SolutionInn App


