Calculate the self-diffusion coefficient in the primitive cubic -Po (Figure 1.23; Figure 1.23 3.36 ) at 500
Question:
Calculate the self-diffusion coefficient in the primitive cubic α-Po (Figure 1.23;
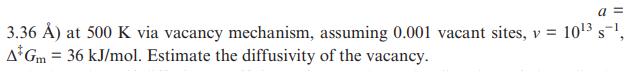
Figure 1.23

Transcribed Image Text:
3.36 ) at 500 K via vacancy mechanism, assuming 0.001 vacant sites, v = 10 s, A Gm = 36 kJ/mol. Estimate the diffusivity of the vacancy. a=
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Equation 310 Equation 318 Po has Nn 6 nearest neighbors and the probability pavail th...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
The drug patch shown in the figure in the next column releases a water-soluble epidermal growth factor (species A) to repair a specific region of wounded tissue on the human body. A slow release of...
-
The Arnold Diffusion Cell shown in Figure 26.5 is a simple device used to measure gas-phase diffusion coefficients for volatile substrates in air. In the present experiment, liquid acetone is loaded...
-
The mass transfer device shown in the figure at the top of the next column is used to carry out the controlled release of a vapor-phase pheromone drug used in pest control. The solid drug sublimes at...
-
Why is ultraviolet light, but not infrared light, effective in making certain materials fluoresce?
-
Determine the moment of inertia and the radius of gyration of the shaded area with respect to the y axis. 10 mm- - 10 mm 50 inm C. 50 mm 10 mm 90 mm
-
Cogan was a long-standing season ticket holder to Toronto Blue Jay baseball games and had made it a practice of allowing his friends and business associatesSherman and Fingoldto purchase some of...
-
(p. 39). Recall that 50 consumers were randomly selected from all consumers in a designated market area to participate in the study. The researchers randomly assigned 25 consumers to read a...
-
Werner Pharmacy, part of a large chain of pharmacies, fills a variety of prescriptions for customers. The complexity of prescriptions filled by Werner varies widely; pharmacists can spend between...
-
Required information The following information applies to the questions displayed below.) Chavez Company most recently reconciled its bank statement and book balances of cash on August 31 and it...
-
Calculate the self-diffusion coefficient of -Po along the direction of the cell edge (Figure 1.23; a = 3.36 ) at 500 K via an interstitial mechanism with full availability of interstitial sites,...
-
Verify the statement that p dir 2 = 2 /6 = a 2 /N n , in which the distance a is the unit-cell edge, the actual jump length, its projection onto the unit cell edge direction, and N n is the...
-
Refrigerant R134a is flowing through a -std type L copper tube in a refrigeration system. In this system, a desuperheater is used to heat water as shown in Figure P4.3. The R134a enters the...
-
Please help. I would really appreciate it. Question 1. Polly owns an electric power plant in the city of Newtown. The market price of electricity in Newtown is $1.00 per kilowatt hour (kwh). Polly's...
-
(Appendix 3A) Jenson Manufacturing is developing cost formula for future planning and cost control. Utilities is one of the mixed costs associated with production. The cost analyst has suggested that...
-
A company has the following trial balance as at 31 December 2015: TRIAL BALANCE AS AT 31 DEC 2015 Dr Cr Sales Revenue 125 000 Purchases 78 000 Carriage 4 000 Electricity and rent 5 100 Administrative...
-
What gets printed to the screen by the following segment of code? String str1 = "hello"; String str2 = "world"; if (!strl.equals(str2)) { System.out.println(str1+" "+str2); } else { }...
-
a) Consider the following financial data (in millions of dollars) for Costello Laboratories over the period of 2014-2018: Year Sales Net income Total assets Common equity 2014 $3,800 $500 $3,900...
-
Test the claim that the population mean = 75, given a sample of n = 100 for which = 78 and s = 15. Test at the = 0.05 significance level.
-
You are standing at x = 9.0 km and your assistant is standing at x = 3.0 km. Lightning bolt 1 strikes at x = 0 km and lightning bolt 2 strikes at x = 12.0 km. You see the flash from bolt 2 at t = 10...
-
Propose the structure of a compound consistent with the following data: (a) C 5 H 10 O, broadband-decoupled 13 C NMR: 7.1, 34.6, 210.5 (b) C 6 H 10 O, broadband-decoupled 13 C NMR: 70.8, 116.2, 134.8
-
Determine the structure of an alcohol with molecular formula C 6 H 14 O that exhibits the following DEPT-135 spectrum: DEPT-135 70 60 50 40 Chemical Shift (ppm) 30 20 10
-
Deduce the structure of a compound with molecular formula C 6 H 14 O 2 that exhibits the following IR, 1 H NMR, and 13 C NMR spectra. 100 80 60 40 20 3000 2500 Wavenumber (cm-1) 4000 3500 2000 1500...
-
question 6 Timely Inc. produces luxury bags. The budgeted sales and production for the next three months are as follows july. august september Sales, in units 1,115. 1229. 1302 Production. in units...
-
On May 12 Zimmer Corporation placed in service equipment (seven-year property) with a basis of $220,000. This was Zimmer's only asset acquired during the year. Calculate the maximum depreciation...
-
Power Manufacturing has equipment that it purchased 7 years ago for $2,550,000. The equipment was used for a project that was intended to last for 9 years and was being depreciated over the life of...

Study smarter with the SolutionInn App


