Crystallographers have a rough rule of thumb that non-H atoms occupy around 18 3 in many
Question:
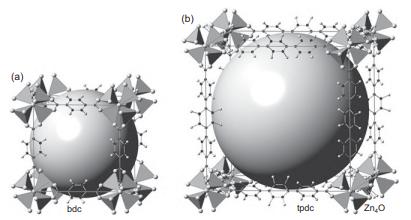
Crystallographers have a rough “rule of thumb” that non-H atoms occupy around 18 Å3 in many crystal structures. Based on this assumption, estimate the percent pore space in the tpdc MOF on the right of Figure 14.14.
Figure 14.14
Transcribed Image Text:
(a) bdc tpdc Zn0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
The contents of the cell shown woul...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
After graduating from business school, George Clark went to work for a Big Six accounting firm in San Francisco. Because his hobby has always been wine making, when he had the opportunity a few years...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The Collectors Choice Inc. ( CCI), a luxury car import company, has an old warehouse at the Port of Miami, Florida, where it temporarily stores expensive sports cars and automotive parts that arrive...
-
For each polynomial function, complete the following in order. (a) Use Descartes rule of signs to determine the different possibilities for the numbers of positive, negative, and nonreal complex...
-
Find the wavelength of the K line in copper (Z = 29) if the wavelength of the K line in iron (Z = 26) is known to be equal to 193 pm.
-
Ms. Yong invested a total of $73,400 in three mutual funds as shown in the following table. The third column shows the change in value of each fund during the subsequent six months. What was the...
-
2. Should the team consider the proposal from Asia General Contractors? Why or why not?
-
The Maser is a new custom-designed sports car. An analysis of the task of building the Maser reveals the following list of relevant activities, their immediate predecessors, and their duration: (a)...
-
Required information (The following information applies to the questions displayed below.) Trey Monson starts a merchandising business on December 1 and enters into the following three inventory...
-
Consider the PtS net listed in Table 1.6. The dehydrated form of MOF-11 has composition Cu 2 (ATC) where ATC is 1,3,5,7-adamantane tetracarboxylate and contains a Cu paddle-wheel unit analogous to...
-
The first peak in the powder diffraction pattern of a mesoporous MCM material with 30 pores recorded with a wavelength of 1.54 is at 2.2 2. Estimate the thickness of the silica walls. A hexagonal...
-
Figure 4 shows the power consumption in the city of San Francisco for a day in September (P is measured in megawatts; t is measured in hours starting at midnight). Estimate the energy used on that...
-
7. A psychiatrist is testing a new ADHD Medication, which seems to have the potentially harmful side effect of increasing the heart rate. For a sample of 50 clinical study participants whose pulse...
-
Determine the type of engagement that your colleague completed for the client. Justify the selected engagement type for the client. Assess the purpose of each financial statement for the client's...
-
Mills Corporation acquired as a long-term investment $235 million of 8% bonds, dated July 1, on July 1, 2024. Company management has classified the bonds as an available-for-sale investment. The...
-
A force of 28 pounds acts on the pipe wrench shown in the figure below. 18 in. 30 (a) Find the magnitude of the moment about O by evaluating ||OA x F||. (0 0 180) Use a graphing utility to graph the...
-
Module 1 1. There has been a rise in cases of measles in RI. The RI Health Department is wondering if the rate of MMR vaccinations has declined since the start of the COVID-19 pandemic. The...
-
Make the best case you can for: a. Parents being entirely responsible for the care of their children. b. Employer-financed day care c. Govermment- financed day care.
-
Marc Company assembles products from a group of interconnecting parts. The company produces some of the parts and buys some from outside vendors. The vendor for Part X has just increased its price by...
-
Give an example of two systems that are in equilibrium with respect to only one of two state variables.
-
Aerobic cells metabolize glucose in the respiratory system. This reaction proceeds according to the overall reaction 6O 2 (g) + C 6 H 12 O 6 (s) 6CO 2 (g) + 6H 2 O(l) Calculate the volume of oxygen...
-
Using acetylene as your only source of carbon atoms, outline a synthesis for 3-hexyne.
-
Callaho Inc. began operations on January 1 , 2 0 1 8 . Its adjusted trial balance at December 3 1 , 2 0 1 9 and 2 0 2 0 is shown below. Other information regarding Callaho Inc. and its activities...
-
Required: 1. Complete the following: a. Colnpute the unit product cost under absorption costing. b. What is the company's absorption costing net operating income (loss) for the quarter? c. Reconcile...
-
Bond Valuation with Semiannual Payments Renfro Rentals has issued bonds that have an 8% coupon rate, payable semiannually. The bonds mature in 6 years, have a face value of $1,000, and a yield to...

Study smarter with the SolutionInn App


