Question: MgF 2 adopts the rutile structure (Figure 1.45) with a = 4.62 and c = 3.04 . The bondvalence parameter R0 MgF = 1.581
MgF2 adopts the rutile structure (Figure 1.45) with a = 4.62 Å and c = 3.04 Å. The bondvalence parameter R0 MgF = 1.581 Å.
(a) Use the bond-valence method to predict the length of the Mg–F bonds.
(b) Use the Born–Mayer equation to estimate the latticeformation energy for MgF2.
(c) Comment on the difference between this value and the value of −2978 kJ/mol obtained from a Born–Haber cycle. Is the agreement between calculated and experimental values similar to that observed for the alkali-metal halides?
(d) Would you expect the lattice-formation energy of rutile (TiO2) to be lower (more stable) than MgF2?
Figure 1.45
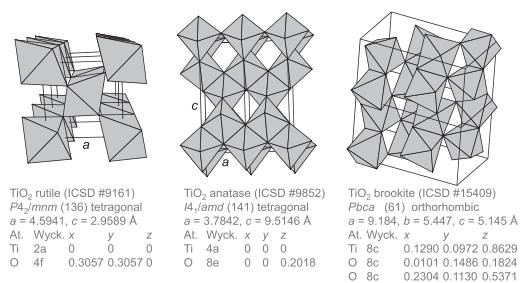
a TiO rutile (ICSD # 9161) P4 /mnm (136) tetragonal a = 4.5941, c = 2.9589 A At. Wyck. x y Ti 2a 0 0 0 4f 0.3057 0.3057 0 FO Z a TiO anatase (ICSD #9852) 14,/amd (141) tetragonal a = 3.7842, c = 9.5146 A At. Wyck. x y z 000 Ti 4a 08e 0 0 0.2018 TiO brookite (ICSD #15409) Pbca (61) orthorhombic a = 9.184, b= 5.447, c= 5.145 A At. Wyck. x y Z Ti 8c O 8c O 8c 0.1290 0.0972 0.8629 0.0101 0.1486 0.1824 0.2304 0.1130 0.5371
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it
a Mg is octahedrally coordinated so the ideal MgF bond valence is 26 13 Using ... View full answer

Get step-by-step solutions from verified subject matter experts


