Question: MO diagrams for cyclic H N molecules are shown in Figure 6.1. (a) Sketch out the MOs and their relative energies for a square H
MO diagrams for cyclic HN molecules are shown in Figure 6.1.
(a) Sketch out the MOs and their relative energies for a square H4 molecule. Determine the number of nodal planes for each MO.
(b) This molecule is prone to a first-order Jahn–Teller distortion. How will the distortion change the shape of the molecule? Redraw the MO diagram after the distortion has taken place.
Figure 6.1
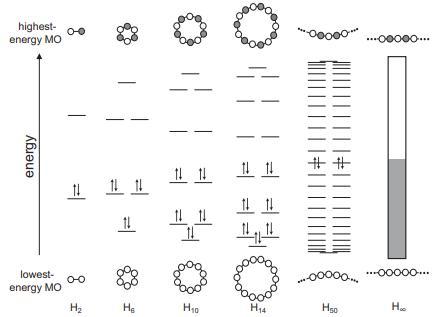
highest- 0-0 energy MO energy lowest- energy MO 0-0 H 14 He 14 14 14 14 11 H0 14 14 H4 --00000 |||||| |||||| ...00000... 00000... ...-00000... Hoo H
Step by Step Solution
3.45 Rating (145 Votes )
There are 3 Steps involved in it
a The MO diagram for a square H 4 molecule is shown below on the le... View full answer

Get step-by-step solutions from verified subject matter experts


