Question: MO diagrams for cyclic H N molecules are shown in Figure 6.1. The AO phases are shown for the lowest- and highest-energy MOs but not
MO diagrams for cyclic HN molecules are shown in Figure 6.1. The AO phases are shown for the lowest- and highest-energy MOs but not for the intermediate MOs.
(a) Sketch out the intermediate MOs indicating the phase of each AO for a cyclic H6 molecule. Determine the number of nodal planes for each of the six MOs.
(b) Repeat part (a) For a cyclic H10 molecule.
Figure 6.1
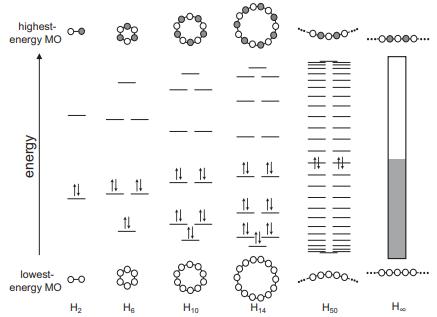
highest- 0-0 energy MO energy lowest- energy MO 00 H2 11 He 11 1 14 14 14 14 11 Ho H14 ooooo. ||||| |||||| "00000.. ooooo... ...ooooo... Hso He
Step by Step Solution
3.37 Rating (147 Votes )
There are 3 Steps involved in it
a The MOs for H 6 are equivalent to the MOs in ben... View full answer

Get step-by-step solutions from verified subject matter experts


