Repeat Exercise 12.47 for a linear chain of eight lithium atoms. Data from Repeat exercise 12.47 The
Question:
Repeat Exercise 12.47 for a linear chain of eight lithium atoms.
Data from Repeat exercise 12.47
The molecular-orbital diagrams for two- and four-atom linear chains of lithium atoms are shown in Figure 12.22. Construct a molecular-orbital diagram for a chain containing six lithium atoms and use it to answer the following questions:
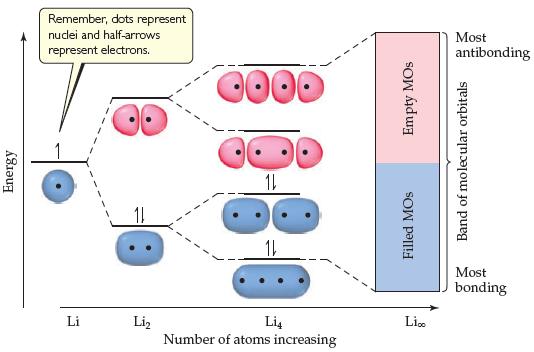
(a) How many molecular orbitals are there in the diagram?
(b) How many nodes are in the lowest energy molecular orbital?
(c) How many nodes are in the highest-energy molecular orbital?
(d) How many nodes are in the highest-energy occupied molecular orbital (HOMO)?
(e) How many nodes are in the lowest-energy unoccupied molecular orbital (LUMO)?
(f) How does the HOMO–LUMO energy gap for this case compare to that of the four- atom case?
Step by Step Answer:

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus





