Question: Prove that Equation (15.6) reduces to the LorenzLorentz equation (15.4) in the case of purely ionic bonding. Equation (15.6) Equation (15.4) a = 4x +
Prove that Equation (15.6) reduces to the Lorenz–Lorentz equation (15.4) in the case of purely ionic bonding.
Equation (15.6)
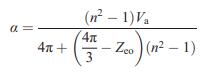
Equation (15.4)
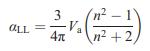
a = 4x + (n - 1) Va 4 3 -Zeo (1)
Step by Step Solution
★★★★★
3.39 Rating (146 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Purely ionic bonding implie... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


