Reciprocal mass susceptibilities Xm for CoO at T = 400 K, 500 K, and 600 K in
Question:
Reciprocal mass susceptibilities
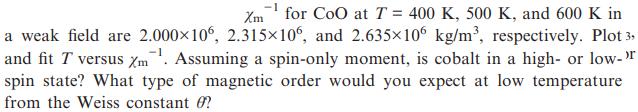
Transcribed Image Text:
Xm for CoO at T = 400 K, 500 K, and 600 K in a weak field are 2.000106, 2.315106, and 2.635106 kg/m, respectively. Plot 3, and fit T versus Xm. Assuming a spin-only moment, is cobalt in a high- or low->r spin state? What type of magnetic order would you expect at low temperature from the Weiss constant ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
S 150 obtained as in the ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
The article "Differences in Susceptibilities of Different Cell Lines to Bilirubin Damage" (K. Ngai, C. Yeung, and C. Leung, Journal of Paediatric Child Health, 2000:36-45) reports an investigation...
-
Explain why the nonlinear susceptibilities for an isotropic medium have the forms given in Eq. (10.25). What are the corresponding forms, in an isotropic medium, of ij klmn and ijklmnp ? Xij =...
-
A particle has wavefunction (x, y, z) = Nze-a(x+ya+z), where N is a normalization constant and er is a positive constant. In this state, which one of the following options represents the eigenvalues...
-
Monroe Inc. is an all-equity firm with 500,000 shares outstanding. It has $2,000,000 of EBIT, and EBIT is expected to remain constant in the future. The company pays out all of its earnings, so...
-
A conical wedge is placed between two horizontal plates that are then slowly moved toward each other. Indicate what will happen to the wedge (a) If μs = 0.20, (b) If μs =...
-
Kim retires from the KLM Partnership on January 1 of the current year. At that time, her basis in the partnership is $75,000, which includes her share of liabilities. The partnership reports the...
-
.000 Flight Att. .40 .24 .39 .13
-
Into which of the three elements of manufacturing cost would each of the following be classified? a. Tubing used in manufacturing bicycles. b. Wages paid by an automobile manufacturer to employees...
-
Product costs and product profitability reports, using a single plantwide factory overhead rate Kao Engines Inc. produces three products pistons , valves, and cams for the heavy equipment industry....
-
What is the percentage of magnetic saturation in an S = paramagnet at 10 K under a field of magnetic induction 1T?
-
Reciprocal mass susceptibility Xmfor (NH4)2Fe(SO4)2 6HO (molar mass 392.139 g/ mol) at T = 100 K, 200 K, and 300 K was measured in a weak field as 1037000kg/m, 2075000 kg/m, and 3112000 kg/m,...
-
Contracting internalizes the problem of information production. Explain what this statement means. (CGA- Canada)
-
In the circuit of Fig. 4-51 write two loop equations using I 1 and I 2 . Then find the currents and node voltages. A 3A ( 4 3 V 792 B +1 D w 392 12 C
-
The capacitor in the circuit shown in Fig. 7-37 has initial charge Q 0 = 800 C, with polarity as indicated. If the switch is closed at t = 0, obtain the current and charge, for t > 0. 100 V (+ 10 4 F
-
A gift shop sells 400 boxes of scented candles a year. The ordering cost is \($60\) for scented candles, and holding cost is \($24\) per box per year. What is the economic order size for scented...
-
Kay Vickery is angry with Gene Libby. He is behind schedule developing supporting material for tomorrows capital budget committee meeting. When she approached him about his apparent lackadaisical...
-
Tharpe Painting Company is considering whether to purchase a new spray paint machine that costs \($3,000\) . The machine is expected to save labor, increasing net income by \($450\) per year. The...
-
A 95% confidence interval for the lives (in minutes) of Kodak AA batteries is 430 < < 470. (See Program 1 of Against All Odds: Inside Statistics.) Assume that this result is based on a sample of...
-
3M Company reports the following financial statement amounts in its 10-K report: a. Compute the receivables, inventory, and PPE turnover ratios for both 2018 and 2017. (Receivables turnover and...
-
Provide a systematic name for each of the following compounds. a. b. c. d. e. H.
-
Aromatic compounds often have multiple names that are all accepted by IUPAC. Provide three different systematic (IUPAC) names for the following compound.
-
For each of the following compounds, draw its structure. a) 2, 6-Dibromo-4-chloroanisole b) Meta-Nitro-phenol
-
On NSE (Indian stock exchange), shares of ICICI Bank trade for 935 rupees. If the spot exchange rate is USD 0.012, what is the no-arbitrage USD price of ICICI Bank ADR? Assume that transactions costs...
-
Income Statement Balance Sheet Balance Sheet Additional Financial Information 1. Market price of Ranfield's common stock: $90.44 at December 31, 2024, and $58.35 at December 31, 2023. 2. Common...
-
There is a credit rating agency for businesses that gives out various amounts of information based on the subscription level. This company is called a. Business Credit Scoring b. Fair Issue c. Dun...

Study smarter with the SolutionInn App


