Reciprocal mass susceptibility Xmfor (NH4)2Fe(SO4)2 6HO (molar mass 392.139 g/ mol) at T = 100 K, 200
Question:
Reciprocal mass susceptibility
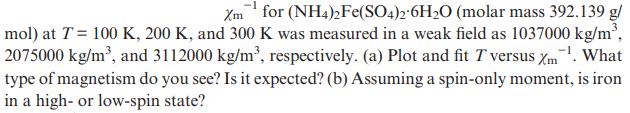
Transcribed Image Text:
Xmfor (NH4)2Fe(SO4)2 6HO (molar mass 392.139 g/ mol) at T = 100 K, 200 K, and 300 K was measured in a weak field as 1037000kg/m, 2075000 kg/m, and 3112000 kg/m, respectively. (a) Plot and fit T versus m. What type of magnetism do you see? Is it expected? (b) Assuming a spin-only moment, is iron in a high- or low-spin state?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a The straight line from 0 K is of a Curie paramagnet ...View the full answer

Answered By

Seema kuldeep
although I don't have an experience of teaching in a particular institute, previously I was an expert on Chegg and I have used to teach my batch mates and also my juniors.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
It is possible to express the magnetic susceptibility ?m in several different units. For the discussion of this chapter, ?m was used to designate the volume susceptibility in SI units, that is, the...
-
It is possible to express the magnetic susceptibility m in several different units. For the discussion of this chapter, m was used to designate the volume susceptibility in SI units, that is, the...
-
Mass susceptibility of Sr 2 MnMoO 6 (molar mass 422.114 g/mol) was measured as a function of temperature, from which the Curie constant, C m , was obtained by leastsquares fitting as C m = 1.30610 4...
-
Given the following information set up the problem in a transportation table and solve for the minimum-cost plan: Minimum total cost? Demand 550 700 750 Capacity 500 500 500 Regular Overtime 50 50...
-
A small screwdriver is used to pry apart the two coils of a circular key ring. The wedge angle of the screwdriver blade is 16° and the coefficient of static friction is 0.12 between the coils and...
-
For each of the following independent situations, determine which partnership(s) (if any) terminate and which partnership(s) (if any) continue. a. The KLMN Partnership is created when the KL...
-
Shared leadership in airplane crews. Human Factors (March 2014) published a study that examined the effect of shared leadership by the cockpit and cabin crews of a commercial airplane. Simulated...
-
Access the financial statements and related disclosure notes of Google Inc. from its website at investor.google.com. In Google's balance sheet, deferred income taxes in 2008 are reported as both an...
-
You have $58,000. You put 16% of your money in a stock with an expected return of 15%, $31,000 in a stock with an expected return of 17%, and the rest in a stock with an expected return of 18%. What...
-
Reciprocal mass susceptibilities Xm for CoO at T = 400 K, 500 K, and 600 K in a weak field are 2.000106, 2.315106, and 2.635106 kg/m, respectively. Plot 3, and fit T versus Xm. Assuming a spin-only...
-
Calculate the value with which you divide molar magnetization M mol in emu/mol in order to get the amount of Bohr magnetons per mole.
-
Decide whether each relation defines y as a function of x. Give the domain and range. y = x 3
-
Brice Looney owns a small retail ice cream parlor. He is considering expanding the business and has identified two attractive alternatives. One involves purchasing a machine that would enable Mr....
-
A positively charged particle initially at rest on the ground moves \(4.0 \mathrm{~m}\) upward in \(2.00 \mathrm{~s}\). If the particle has a chargeto-mass ratio of \(10 \mu \mathrm{C} / \mathrm{g}\)...
-
Central States Telecom provides communication services in Iowa, Nebraska, the Dakotas, and Montana. Central States purchased goodwill as part of the acquisition of Sheldon Wireless Company, which had...
-
Shown below is selected information from the financial records of Merris Corporation as of December 31: Required a. Determine which of the above items will appear on the statement of cash flows and...
-
Pippa runs a photographic studio specializing in black and white portrait photography. Clients book a one hour studio session and are entitled to receive two large photographs of their choice from...
-
In many of the preceding examples, confidence intervals are used toward the ultimate goal of estimating the value of a population parameter. Confidence intervals can also be used as a...
-
The following T-accounts show postings of selected transactions. Indicate the journal used in recording each of these postings a through e. Cash Accounts Receivable Inventory (d) 500 (e) 300 (b)...
-
In Chapter 9, we saw that meta-chloroperoxybenzoic acid (MCPBA) is a peroxy acid commonly used to convert alkenes into epoxides. Recall that peroxy acids have the following structure a) Draw the...
-
Provide at least five different acceptable IUPAC names for the following compound.
-
Compound A has molecular formula C 8 H 8 . When treated with excess Br 2 , compound A is converted into compound B, with molecular formula C 8 H 8 Br 2 . Identify the structures of compounds A and B....
-
Current Attempt in Progress On July 3 1 , 2 0 2 2 , Crane Compary had a cash balance per books of $ 6 , 2 4 5 . 0 0 . The statement from Dakata State Bark on that date showed a balance of $ 7 , 7 9 5...
-
Cede & Co. expects its EBIT to be $89,000 every year forever. The firm can borrow at 5 percent. Cede currently has no debt, and its cost of equity is 10 percent. If the tax rate is 35 percent, what...
-
In the Marriott example, one discussion point considered when a firm might use a single hurtle rather than different divisional or business unit rates. When a single rate is used and the divisions...

Study smarter with the SolutionInn App


