In Chapter 9, we saw that meta-chloroperoxybenzoic acid (MCPBA) is a peroxy acid commonly used to convert
Question:
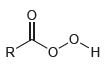
a) Draw the structure of MCPBA.
b) Provide a systematic name for the compound formed by replacing the chlorine atom in MCPBA with a methyl group.
Transcribed Image Text:
н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
a b 3methylp...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the structure of an alkyne that can be converted into 3-ethylpentane upon hydrogenation. Provide a systematic name for this compound.
-
In Chapter 9, we considered the dimensions of the double helix (see Figure 9.15). In an helix of a protein, there are 3.6 amino acids per complete turn. Each amino acid advances the helix by 0.15...
-
In Chapter 9, we learned about addition of water across a Ï bond. Identify whether the alkene has been oxidized, reduced, or neither. (First look at each carbon atom separately, and then look at...
-
2. Evaluate the following definite integrals (a) (2x - 6x) dx /6 (b) 16 cos 3t + 2 sin 3t dt x+1 2 da
-
A sample of soil was tested in the laboratory and the following grain size analysis results were obtained: Atterberg limits on minus No. 40 material were: LL = 62, PL = 20. Determine the USCS letter...
-
On Form W-4, Virginia decided to request her employer withhold more taxes than she generally would have withheld. a. Is it permissible to request greater withholdings than an individual is entitled...
-
16. What is an appropriation? How can budgetary approval be arranged to give the legislative body maximum control over the budget? How can it be arranged to give the executive maximum flexibility?
-
Indicate how the following items are recorded in the accounting records in the current year of Coronet Co. (a) Impairment of goodwill. (b) A change in depreciating plant assets from accelerated to...
-
Exercise 5 - 1 1 ( Algo ) Missing Data; Basic CVP Concepts [ L 0 5 - 1 , LO 5 - 9 ] Fill in the missing amounts in each of the eight case situations below. Each case is independent of the others....
-
Innovation Company is thinking about marketing a new software product. Upfront costs to market and develop the product are $5 million. The product is expected to generate profits of $1 million per...
-
What is removed during each of the three stages of wastewater treatment: primary, secondary, and tertiary? During which state would you expect items to be recovered that were accidentally flushed,...
-
Provide at least five different acceptable IUPAC names for the following compound.
-
Use Figure 6.17 and the fact that F(2) = 3 to sketch the graph of F(x). Label the values of at least four points. Area = 2 frie + F'(x) 12 3 4 5 6 7 Area = 7 Figure 6.17 Area = 4 +x 8
-
Part 1 of 4 05 points abook Print References Required information Problem 24-2A (Algo) Payback period, accounting rate of return, net present value, and net cash flow calculation LO P1, P2, P3 [The...
-
Keenan Music's CEO has been pondering about the recent proposal of the Specialty Guitar Project. The accountant has done a capital budgeting analysis on the project and outlined the conditions that...
-
On July 1, 2025, Sheridan Co. pays $15,000 to Blue Spruce Insurance Co. for a 2-year insurance contract. Both companies have fiscal years ending December 31. (a1) Journalize the entry on July 1 and...
-
A CU triaxial test with c = 20 psi is performed on a sand and a deviator stress of 80 psi fails the specimen. Previous tests revealed that the effective friction angle for this sand is 35. Calculate...
-
Haliburton Mills Inc. is a large producer of men's and women's clothing. The company uses standard costs for all of its products. The standard costs and actual costs for a recent period are given...
-
Find the area of each figure. Express it as a polynomial in descending powers of the variable x. Refer to the formulas at the back of this text if necessary. x +8 x 2 + 8
-
From a medical tourist perspective, compare Shouldice with the traditional hospital in terms of the key factors of competition. Using Table 15-3, why would Shouldice attract patients from outside the...
-
What is the relationship between the specific rotations of (2R, 3R)-dichloro- pentane and (2S, 3S)-dichloro pentane? Between (2R, 35)-dichloro pentane and (2R, 3R)-dichloro pentane?
-
What is the stereo chemical configuration of the enantiomer of (2S, 4R)-2, 4-oclanediol?
-
What are the stereo chemical configurations of the two diastereomers of (25, 4R) -2, 4-octanediol?
-
Suppose I have computed the cost of carbon per mile for my car at 0 . 0 1 2 per mile. Assume that the interest rate is 4 % and that I drive the car 2 8 , 0 0 0 miles per year. What is the present...
-
Imagine that in stable growth period, the firm earns ROIC of 10% and has after tax EBIT of 200 and reinvestment $ of 40. What is the steady state growth rate? 20% O 10% 2%
-
Tanner-UNF Corporation acquired as a long-term investment $160 million of 5.0% bonds, dated July 1, on July 1, 2021. Company management has the positive intent and ability to hold the bonds until...

Study smarter with the SolutionInn App


