Question: The table below contains data for the alkali-metal fullerides A 3 C 60 . (a) Give a brief explanation for the T c changes observed
The table below contains data for the alkali-metal fullerides A3C60.
(a) Give a brief explanation for the Tc changes observed in the 0 GPa data.
(b) Give a brief explanation for the pressure dependence of Tc in Rb3C60.
(c) Comment on differences in Tc between the two series.
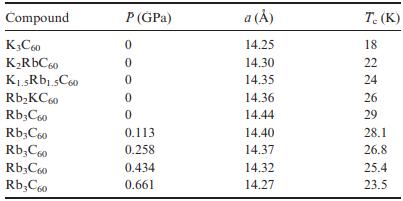
Compound K3C60 KRbC60 K.5Rb1.5C60 RbKC60 Rb3C60 Rb3C60 RbC60 Rb3C60 RbC60 P (GPa) 0 0 0 0 0 0.113 0.258 0.434 0.661 a () 14.25 14.30 14.35 14.36 14.44 14.40 14.37 14.32 14.27 T. (K) 18 22 24 26 29 28.1 26.8 25.4 23.5
Step by Step Solution
3.52 Rating (155 Votes )
There are 3 Steps involved in it
abc The data relate to Figure 1210 where the dependence of T c on the volume per C 60 was presented ... View full answer

Get step-by-step solutions from verified subject matter experts


