Question: The table below lists the in-plane conductivity for graphite and K 0.125 C at two different temperatures. Comment on these values. Substance Graphite K0.125C (90
The table below lists the in-plane conductivity for graphite and K0.125C at two different temperatures. Comment on these values.
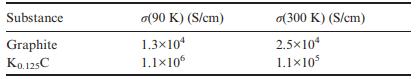
Substance Graphite K0.125C (90 K) (S/cm) 1.3x104 1.1106 o(300 K) (S/cm) 2.510 1.110
Step by Step Solution
3.45 Rating (158 Votes )
There are 3 Steps involved in it
The potassium intercalate has a higher conductivity than graphite at both temperatures This ca... View full answer

Get step-by-step solutions from verified subject matter experts


