Use the ClausiusMossotti expression in Equation (8.13) and Table 8.2 to estimate permittivities of SnO 2 (rutile-type,
Question:
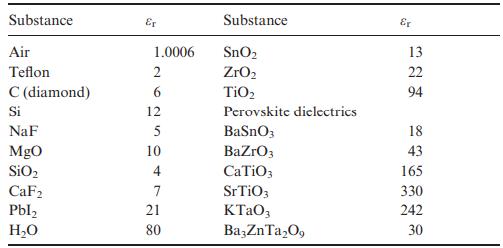
Use the Clausius–Mossotti expression in Equation (8.13) and Table 8.2 to estimate permittivities of SnO2 (rutile-type, P42/mnm, Z = 2, a = 4.74 Å, c = 3.19 Å), TiO2 (rutile-type, a = 4.59 Å, c = 2.96 Å) and ZrO2 (baddeleyite-type, P21/c, Z = 4, unit-cell volume = 141 Å). Does the Clausius–Mossotti equation give a reasonably accurate estimate for each compound when compared to the experimental values of εr given in Table 8.1? If not, what is the origin of the discrepancy?
Equation (8.13)
![a= V 4 2 Er (+2) [CGSes : a in , V, in A]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1705/9/2/1/12165ae4a61b74551705921119175.jpg)
Table 8.2
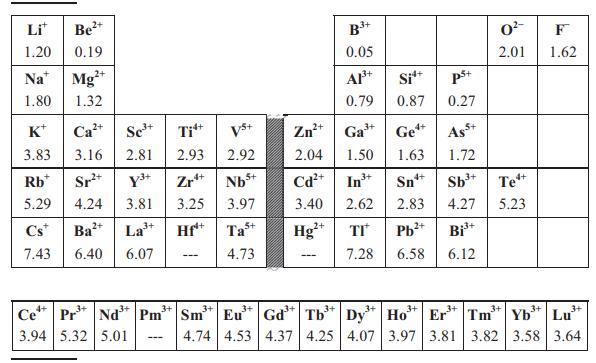
Table 8.1
Transcribed Image Text:
a= V 4 2 Er (+2) [CGSes : a in , V, in A]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Calculated values are r 13 SnO 2 r 20 ZrO 2 and r 43 TiO 2 Th...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
A researcher wanted to find out if there was difference between older movie goers and younger movie goers with respect to their estimates of a successful actors income. The researcher first...
-
Can you help assist me with an outline for the following essay on supervisory role with the following topics being addressed. I am just looking for an outline as my starting point. THANKS If given...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
ABC Insurance Company has issued a commercial package policy to the Henderson Company. ABC recently discovered that company executives misrepresented important information about the business to...
-
As shown in Fig.P2.39, a steel wire suspended vertically having a cross-section area A and an initial length X0 is stretched by a downward force F applied to the end of the wire. The normal stress in...
-
Consider a without replacement sample of size 2 from a population of size 4, with joint inclusion probabilities 12 = 34 = 0.31, 13 = 0.20, 14 = 0.14, 23 = 0.03, and 24 = 0.01. a. Calculate the...
-
Going online for health information. A cyberchondriac is defined as a person who regularly searches the Web for health care information. A Harris Poll surveyed 1,010 U.S. adults by telephone and...
-
Suppose you get a job at Aoki Corporation, a firm that manufactures glass for industrial and consumer markets. Aoki is a large firm but has little international experience. Senior managers are...
-
Mice 2 Fall 2020 reused 9 On December 31, 2020, SachsCorp had outstanding 600.000 shares of common stock and 50.000 shares of 8% cumulative preferred stock (par $40). 20 April 30, 2021, issued an...
-
Why doesthe polarizability of the lanthanoid ions decrease as the atomic numberincreases?
-
Derive the ClausiusMossotti equation for the CGSes system of units.
-
Suppose the economy starts with output at potential. Then a supply shock occurs: oil prices rise sharply. The Fed is partly accommodative: it raises the real interest rate, but not by enough to keep...
-
Explain why its important to study management.
-
Wildhorse has not logged since 2016. If Wildhorse logged and sold 1,062,000 board feet of timber in 2027, when the timber cruise (appraiser) estimated 5,900,000 board feet, determine the cost of...
-
Y = AK[1-a R P = QAKa-1[1-a W P = (1 -Q) AKL-a 1= 14 1 -4 Y = C
-
Inferring Transactions from Financial Statements (FSET) Wired.com Inc. is a large e-commerce company, with over $31 billion in revenues for the fiscal year ended December 31, 20X2. For the year ended...
-
Finding Standard Deviation from a Frequency Distribution. In Exercises 37-40, refer to the frequency distribution in the given exercise and compute the standard deviation by using the formula below,...
-
Suppose that when a company has 5 customer service reps (CSRs) in its call centre at any given time, they collectively serve 40 customers per hour on average. If there are 5 CSRs, what is the...
-
What are conversion costs? What are prime costs?
-
Discuss the following statement: If the temperature of the system increased, heat must have been added to it.
-
Oxygen reacts with solid glycylglycine C 4 H 8 N 2 O 3 to form urea CH 4 N 2 O, carbon dioxide, and water: 3O 2 (g) + C 4 H 8 N 2 O 3 (s) CH 4 N 2 O(s) + 3CO 2 (g) + 2H 2 O(l) At T = 298 K and 1.00...
-
Identify the reagents that you would use to achieve each of the following transformations: a. b. Br Br
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?
-
NEED THIS EXCEL TABLE ASAP PLEASE!!!! Presupuesto Operacional y C lculo del COGS Ventas Proyectadas: Ventas Proyectadas: $ 4 5 0 , 0 0 0 Precio por unidad: $ 4 5 0 Unidades vendidas: 4 5 0 , 0 0 0 4...
-
The wash sale rules apply to disallow a loss on a sale of securities_______? Only when the taxpayer acquires substantially identical securities within 30 days before the sale Only when the taxpayer...

Study smarter with the SolutionInn App


