When dimethylbenzenes (xylenes) are passed over acidic zeolites (HZSM-5 that contains 10-rings and HY that contains larger
Question:
When dimethylbenzenes (xylenes) are passed over acidic zeolites (HZSM-5 that contains 10-rings and HY that contains larger 12-rings), two processes can occur: isomerization to different mixtures of ortho, meta, and para isomers; or disproportionation to toluene (methylbenzene) and trimethylbenzenes, as shown in the table. State which of the shape-selective catalysis effects shown in Figure 14.7 is responsible for the enhanced para- to ortho-selectivity of HZSM-5 and for favoring the isomerization reaction over disproportionation in HZSM-5.

Figure 14.7
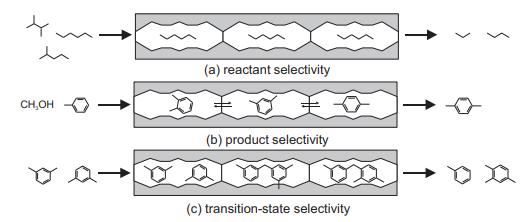
Transcribed Image Text:
Zeolite HZSM-5 HY para-lortho-selectivity 2.9 1.0 Isomerization/disproportionation 33 1.5 in
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
HY is a largepore zeolite Similar amounts of isomeri...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
The Taos Museum of Southwestern Arts and Crafts (TMSAC) presents rotating exhibits of the works of artists and artisans from the Southwestern United States. Historically, the museum has derived its...
-
Project the 2 4-1 IV design in Example 8-1 into two replicates of a 2 2 design in the factors A and B. Analyze the data and thaw conclusions. Example 8-1: Consider the filtration rate experiment in...
-
TIM produces and sells two products, the MK and the KL. The organisation expects to sell 2 MK for every 2 KLs and have monthly sales revenue of GHe150,000. The MK has a C/S ratio of 20% whereas the...
-
What is the smallest (most negative) 32-bit binary number that can be represented with (a) Unsigned numbers? (b) Twos complement numbers? (c) Sign/magnitude numbers?
-
A beam of light of frequency w, equal to the resonant frequency of transition of atoms of gas, passes through that gas heated to temperature T. In this case hw >> kT. Taking into account induced...
-
Evaluate the answers accurate to the cent. $500 (1 0.05)2
-
4. What should Maggie, Paul, and Steve do? In explaining your answer, address the concerns of each of the three team members.
-
Refer to the Auditing in Practice feature What Was He Thinking? An Example of Poor Professional Judgment and Low Audit Quality. a. Briefly explain the common themes indicating poor professional...
-
cash Jenkins Company decided to trade in an old machine for a new one. The old machine originally cost $68,000, and on the date it was traded in, it had an accumulated depreciation of $40,600. The...
-
The first peak in the powder diffraction pattern of a mesoporous MCM material with 30 pores recorded with a wavelength of 1.54 is at 2.2 2. Estimate the thickness of the silica walls. A hexagonal...
-
Haag and co-workers measured the cracking rate of n-hexane by HZSM-5 relative to a high-surface-area catalyst under identical experimental conditions. The dependence of the rate on the Al:Si ratio...
-
Identify four people who have contributed to the theory and techniques of operations management.lop4
-
At March 31, account balances after adjustments for Vizzini Cinema are as follows: Account Balances Accounts Cash Supplies Equipment (After Adjustment) $11,000 4,000 50,000 Accumulated...
-
2. "A student holds a thin aluminum pie pan horizontally 2 m above the ground and releases it. Using a motion detector, she obtains the graph shown in Figure P3.12. Based on her measurements, (a)...
-
Mark has two sticks, 25 inches, and 20 inches. If he places them end-to-end perpendicularly, what two acute angles would be formed when he added the hypotenuse?
-
A wedding website states that the average cost of a wedding is $29,205. One concerned bride hopes that the average is less than reported. To see if her hope is correct, she surveys 36 recently...
-
2. (10 pts each) Use partial fractions decomposition and the tables to find the inverse z- transform of each of the following: a. X(z)= 6z-z z3-4z2-z+4 4z2 b. G(z)=- (z-1) (z-0.5) 3z +1 c. X(z) =...
-
When the Family and Medical Leave Act was passed in the United States in 1993, it was attacked as overly generous by some and as inadequate by others. Discuss the arguments behind each of these views.
-
The Pletcher Transportation Company uses a responsibility reporting system to measure the performance of its three investment centers: Planes, Taxis, and Limos. Segment performance is measured using...
-
Identify the reagents you would use to achieve each of the following transformations: (a) Conversion of all of the carbon atoms in bromoethane into CO 2 gas (b) Conversion of all of the carbon atoms...
-
An athlete at high performance inhales ~3.75 L of air at 1.0 atm and 298 K at a respiration rate of 32 breaths per minute. If the exhaled and inhaled air contain 15.3 and 20.9% by volume of oxygen,...
-
The temperature of 1.75 moles of an ideal gas increases from 10.2C to 48.6C as the gas is compressed adiabatically. Calculate q, w, U, and H for this process assuming that C V ,m = 3/2 R.
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Mrquered Mrquered
-
You plan to invest $10,00 today in an investment account earning 5% interest. You then plan to invest an additional $1,000 into this account each year for the next twenty years. How much money will...

Study smarter with the SolutionInn App


