Question: Fill in the missing steps from (4.9.30) to (4.9.31). First, write a Sommerfeld expansion to second order in T for the total electron number N.
Fill in the missing steps from (4.9.30) to (4.9.31). First, write a Sommerfeld expansion to second order in T for the total electron number N. The lowest-order terms of both U and N can then be expanded as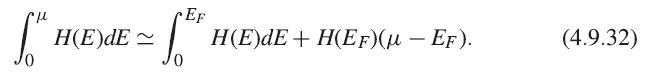
Using the constraint that N is constant for all temperatures, you can obtain a relation for μ in terms of T and substitute this into the equation for U, and then take its derivative, keeping terms to lowest order in T.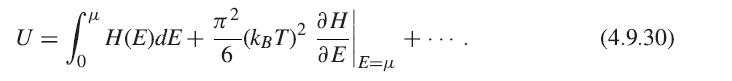
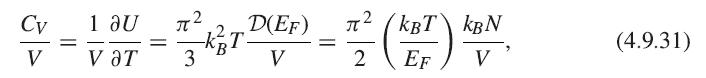
[th B EF H(E)DE ~ ~ f H(E)dE+ H(EF)( Ef). (4.9.32)
Step by Step Solution
3.51 Rating (154 Votes )
There are 3 Steps involved in it
The number of electrons is equal to N NEDE dE 4919 Following the same logic as equations 49254930 ... View full answer

Get step-by-step solutions from verified subject matter experts


