The observed value of (gamma), see equation (8.3.6), for sodium is (4.3 times 10^{-4} mathrm{cal} mathrm{mole}^{-1} mathrm{~K}^{-2}).
Question:
The observed value of \(\gamma\), see equation (8.3.6), for sodium is \(4.3 \times 10^{-4} \mathrm{cal} \mathrm{mole}^{-1} \mathrm{~K}^{-2}\). Evaluate the Fermi energy \(\varepsilon_{F}\) and the number density \(n\) of the conduction electrons in the sodium metal. Compare the latter result with the number density of atoms (given that, for sodium, \(ho=0.954 \mathrm{~g} \mathrm{~cm}^{-3}\) and \(M=23\) ).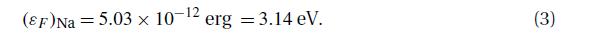
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





