Consider a mixture of molecules of type A and B to which a small amount of type
Question:
Consider a mixture of molecules of type A and B to which a small amount of type C molecules is added. Assume that the Gibbs free energy of the resulting tertiary system is given by
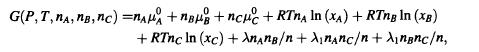
= where n = n+ng+nc, nc nA, and nc
The quantities (P,T), =(P,T), and = (P,T) are the chemical potentials of pure A, B, and C, respectively, at pressure P and temperature T. For simplicity, assume that ==
c. To lowest order in the mole fraction xc, compute the shift in the critical temperature and critical mole fraction of A due to the presence of C.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:






