Radiocarbon dating: Carbon-14 is a radioactive isotope of carbon that decays by emitting a beta particle. In
Question:
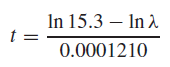
An archaeologist finds a small piece of charcoal from an ancient campsite. The charcoal contains 1 g of carbon.
a. Unknown to the archaeologist, the charcoal is 11,000 years old. What is the true value of the emission rate λ̂?
b. The archaeologist plans to count the number X of emissions in a 25 minute interval. Find the mean and standard deviation of X.
c. The archaeologist then plans to estimate λ with λ̂ = X/25. What is the mean and standard deviation of λ̂?
d. What value for λ̂ would result in an age estimate of 10,000 years?
e. What value for λ̂ would result in an age estimate of 12,000 years?
f. What is the probability that the age estimate is correct to within ±1000 years?
DistributionThe word "distribution" has several meanings in the financial world, most of them pertaining to the payment of assets from a fund, account, or individual security to an investor or beneficiary. Retirement account distributions are among the most...
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





