In the oil industry, water that mixes with crude oil during production and transportation must be removed.
Question:
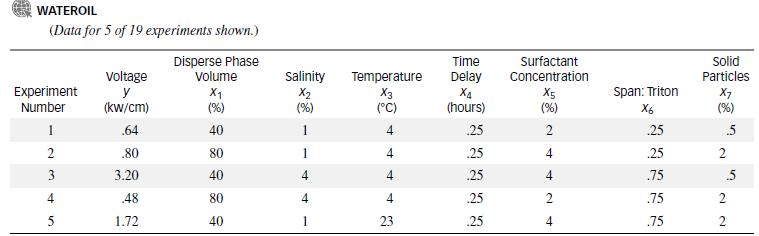
In the oil industry, water that mixes with crude oil during production and transportation must be removed. Chemists have found that the oil can be extracted from the water/oil mix electrically. Researchers at the University of Bergen (Norway) conducted a series of experiments to study the factors that influence the voltage (y) required to separate the water from the oil. (Journal of Colloid and Interface Science, Aug. 1995.) The seven independent variables investigated in the study are listed in the table below. (Each variable was measured at two levels—a “low” level and a “high” level.) Sixteen water/oil mixtures were prepared using different combinations of the independent variables; then each emulsion was exposed to a high electric field. In addition, three mixtures were tested when all independent variables were set to 0. The data for all 19 experiments are saved in the WATEROIL file. (The first 5 experiments are listed in the next table.)
a. Propose a first-order model for y as a function of all seven independent variables.
b. Use a statistical software package to fit the model to the data in the table.
c. Fully interpret the β estimates.
d. Assess model adequacy by conducting the F test, interpreting Ra2, and interpreting 2s.
e. Consider the model, E(y) = βo + β1x1 + β2x2 + β3x5. The researchers concluded that “in order to break a water-oil mixture with the lowest possible voltage, the volume fraction of the disperse phase (x1) should be high, while the salinity (x2) and the amount of surfactant (x5) should be low.” Use this information to find a 95% prediction interval for this “low” voltage y. Interpret the interval.
Step by Step Answer:

Statistics For Engineering And The Sciences
ISBN: 9781498728850
6th Edition
Authors: William M. Mendenhall, Terry L. Sincich





