Question: A compound with m.p. = -22C has a parent peak in its mass spectrum at m/z = 113. The 1 H NMR spectrum shows absorptions
A compound with m.p. = -22°C has a parent peak in its mass spectrum at m/z = 113. The 1H NMR spectrum shows absorptions at δ = 1.2 (t, 3 H), 3.5 (s, 2 H), and 4.2 (q, 2 H). The IR spectrum exhibits significant bands at v˜ = 3000, 2250, and 1750 cm-1. What is its structure?
(a)
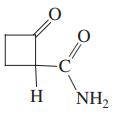
(b)
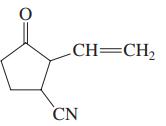
(c)
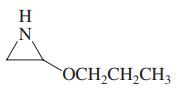
(d)
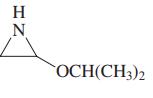
(e) NCCH2CO2CH2CH3
-C' H NH2
Step by Step Solution
3.38 Rating (170 Votes )
There are 3 Steps involved in it
Given that m7 value 113 By option verification option A ... View full answer

Get step-by-step solutions from verified subject matter experts


