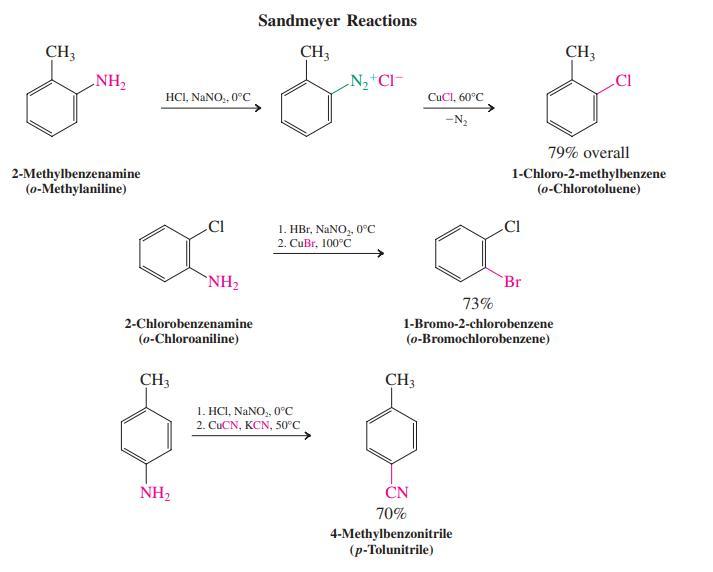
As mentioned in Section 22-10, the Sandmeyer reactions, in which copper(I) ion catalyzes substitution of the nitrogen
Question:
As mentioned in Section 22-10, the Sandmeyer reactions, in which copper(I) ion catalyzes substitution of the nitrogen in arenediazonium salts by Cl, Br, or CN, take place by complex mechanisms involving radicals. Explain why these substitutions do not follow either the SN1 or SN2 pathway.
Section 22-10
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:




