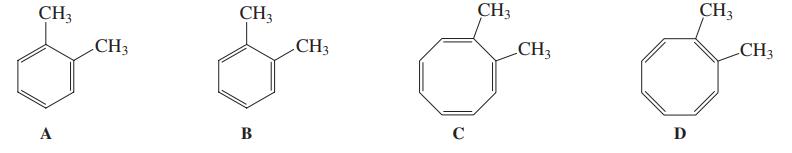
Because of cyclic delocalization, structures A and B shown here for o-dimethylbenzene (o-xylene) are simply two resonance
Question:
Because of cyclic delocalization, structures A and B shown here for o-dimethylbenzene (o-xylene) are simply two resonance forms of the same molecule. Can the same be said for the two dimethyl-cyclooctatetraene structures C and D? Explain.
Transcribed Image Text:
CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH3 A В C D
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
As the structure a and b are two canonical forms of xylene but this is not true in c...View the full answer

Answered By

Saher bibi
I have done my Master of philosophy in the field of chemistry of natural products my major was (Isolation, Identification and Bioevaluation of natural products from medicinal plant) from Quaid-i-Azam University Islamabad, Pakistan, ranked as No. 1 university of Pakistan. I got scholarships and a laptop from the government of Pakistan on the basis of good academic performance
I am proficient in identification and screening of bioactive phytochemicals using HPLC-MS & MS/MS techniques. My M.phil research work consisted of identification of phytoconstituents by using HPLC MS/MS profiling, 80% of the compounds from the crude extract has been identified without the need for purification, which makes it a useful tool for screening and dereplication. Isolation and structure elucidation of secondary metabolites from Medicago Polymorpha for drug discovery using a combination of MS and NMR techniques. Identification of the metabolites involves the spectroscopic techniques using NMR, MS, GCMS, UV-VIS, and classical wet chemical techniques. Troubleshoot and maintenance of analytical instruments as needed. Structure elucidation of novel natural products from Medicago Polymorpha by using NMR spectroscopy, LCMS, isolation and purification of compounds by using silica gel chromatography.
I have experience of teaching at university of Wah. I have taught different courses such as inorganic chemistry, biochemistry and general chemistry to BS 4th and BS 2nd. I have taught these courses in funny and creative manner. Several people think that chemistry is rough and tough subject but i want to say no its not like that because no subject becomes difficult when teacher makes it easy. All those who are facing difficulty in studying chemistry should come to me and see how i am making chemistry easy. thanks.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
(a) Describe the molecule xenon trioxide, XeO3, using four possible Lewis structures, one each with zero, one, two, or three Xe-O double bonds. (b) Do any of these resonance structures satisfy the...
-
The following three Lewis structures can be drawn for N2O: (a) Using formal charges, which of these three resonance forms is likely to be the most important? (b) The N-N bond length in N2O is 1.12...
-
The reaction of two equivalents of Mg with 1, 4-dibromobutane produces compound A. The reaction of A with two equivalents of CH3CHO (acetaldehyde). followed by work-up with dilu aqueous acid,...
-
Describe the typical terrorist cell.
-
Describe what happened during the 1970s to spur the growth in project management and compare and contrast this with the events that occurred during the 1990s and that are occurring today.
-
Show the factorization and solve. 4x 1 + 2x 2 + 4x 3 = 20 2x 1 + 2x 2 + 3x 3 + 2x 4 = 36 4x 1 + 3x 2 + 6x 3 + 3x 4 = 60 2x 2 + 3x 3 + 9x 4 = 122
-
Distinguish between direct and indirect discrimination? LO1
-
Below are certain events that took place at Hazzard, Inc. , last year: a. Short-term investment securities were purchased. b. Equipment was purchased. c. Accounts payable increased. d. Deferred taxes...
-
What is the net cash flow from financing activities for 2018
-
Giovo was in an accident with McDonald. McDonalds insurer, GEICO, and Giovos attorney exchanged offers and counteroffers to settle Giovos negligence claim. GEICO eventually agreed to meet all of...
-
Vanillin, whose structure is shown in the margin and is the subject of the Chapter Opening, is a benzene derivative with several functional groups, each one of which displays its characteristic...
-
The energy levels of the 2-propenyl (allyl) and cyclopropenyl p systems (see margin) are compared qualitatively in the diagram below. (a) Draw the three molecular orbitals of each system, using plus...
-
The figure is a drawing of a 3- by 18-mm latching spring. A preload is obtained during assembly by shimming under the bolts to obtain an estimated initial deflection of 2 mm. The latching operation...
-
1. Why did the Iconoclast emperors believe that using images in worship was wrong? 2. How are recent examples of iconoclasm similar to those of the early medieval period? 3. Why is iconoclasm a...
-
1. Difference Between Essential and Non-Essential Nutrients 2. what is Conditionally Essential Nutrients? explain with examples
-
1. what is the Signs of Malnutrition?
-
Example 5.1 Simply Supported Truss 8' B 2 kips Determine: a) Support reactions b) Internal force in each member Pin 5' D 4 kips 7' 6' Roller
-
Find the rank by reducing to normal form: 1 1 2 3 1 3 0 3 1 -2-3-31 1 12 3
-
Wang Company began operations on January 1, Year 1, by issuing common stock for $70,000 cash. During Year 1, Wang received $88,000 cash from revenue and incurred costs that required $65,000 of cash...
-
Prepare a stock card using the following information A company is registered for GST which it pays quarterly, assume GST was last paid on the 30th of June 2019. It uses weighted average cost...
-
Explain how X-ray diffraction can be used to determine the helical configuration of biological molecules.
-
Describe the characteristics of the Fermi-Dirac distribution. Why is it appropriate to call the parameter u. a chemical potential?
-
Equivalent lattice points within the unit cell of a Brava is lattice have identical surroundings. What points within a body-centred cubic unit cell are equivalent to the point (1/2, 0, 1/2)?
-
a Campbell Inc. produces and sells outdoor equipment. On July 1, 2011. Campbell issued $40,000,000 a 10-year, 10% bonds at a market (effective) interest rate of 9%, receiving Cash of 548,601,480....
-
A company has net income of $196,000, a profit margin of 9.7 percent, and an accounts receivable balance of $135,370. Assuming 70 percent of sales are on credit, what is the companys days sales in...
-
The AICPA guidelines suggest that taxes should be transparent and visible. This means that: a. The taxes affect similarly situated taxpayers in a similar manner. b. Taxes should be due at the same...

Study smarter with the SolutionInn App


