The energy levels of the 2-propenyl (allyl) and cyclopropenyl p systems (see margin) are compared qualitatively in
Question:
The energy levels of the 2-propenyl (allyl) and cyclopropenyl p systems (see margin) are compared qualitatively in the diagram below. (a) Draw the three molecular orbitals of each system, using plus and minus signs and dotted lines to indicate bonding overlap and nodes, as in Figure 15-4. Does either of these systems possess degenerate molecular orbitals? (b) How many p electrons would give rise to the maximum stabilization of the cyclopropenyl system, relative to 2-propenyl (allyl)? (Compare Figure 15-5, for benzene.) Draw Lewis structures for both systems with this number of p electrons and any appropriate atomic charges. (c) Could the cyclopropenyl system drawn in (b) qualify as being “aromatic”? Explain.
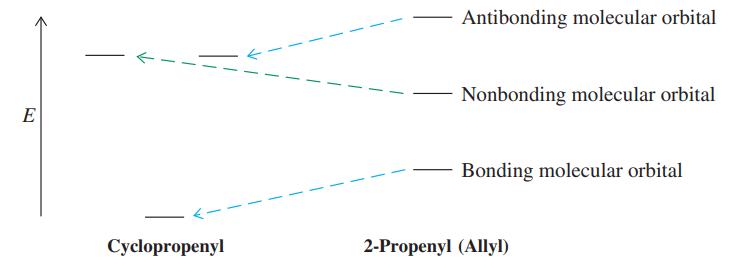
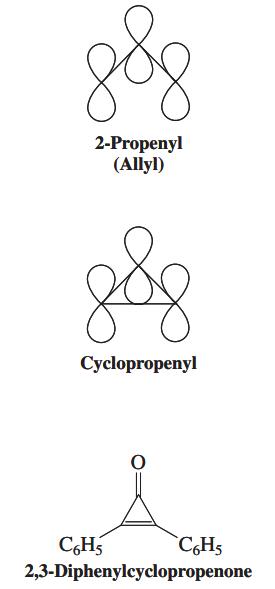
Figure 15-4
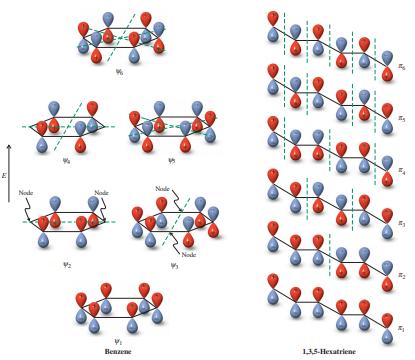
Figure 15-5
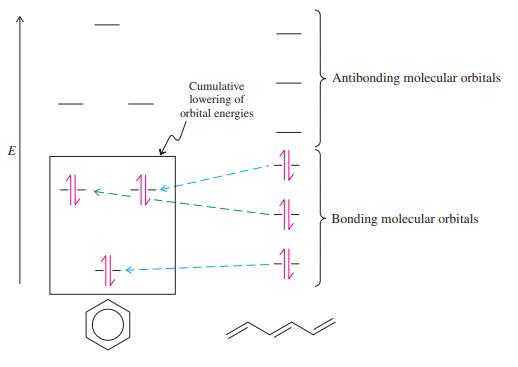
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore





