Biochemical oxidation of aromatic rings is catalyzed by a group of liver enzymes called aryl hydroxylases. Part
Question:
Biochemical oxidation of aromatic rings is catalyzed by a group of liver enzymes called aryl hydroxylases. Part of this chemical process is the conversion of toxic aromatic hydrocarbons such as benzene into water-soluble phenols, which can be easily excreted. However, the primary purpose of the enzyme is to enable the synthesis of biologically useful compounds, such as the amino acid tyrosine.
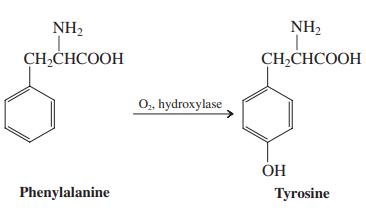
(a) Extrapolating from your knowledge of benzene chemistry, which of the following three possibilities seems most reasonable: The oxygen is introduced by electrophilic attack on the ring; the oxygen is introduced by free-radical attack on the ring; or the oxygen is introduced by nucleophilic attack on the ring?
(b) It is widely suspected that oxacyclopropanes play a role in arene hydroxylation. Part of the evidence is the following observation: When the site to be hydroxylated is initially labeled with deuterium, a substantial proportion of the product still contains deuterium atoms, which have apparently migrated to the position ortho to the site of hydroxylation.
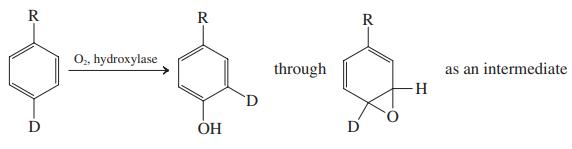
Suggest a plausible mechanism for the formation of the oxacyclopropane intermediate and its conversion into the observed product.
Assume the availability of catalytic amounts of acids and bases, as necessary. In victims of the genetically transmitted disorder called phenylketonuria (PKU), the hydroxylase enzyme system described here does not function properly. Instead, phenylalanine in the brain is converted into 2-phenyl-2-oxopropanoic (phenylpyruvic) acid, the reverse of the process shown in Problem 57 of Chapter 21. The buildup of this compound in the brain can lead to severe retardation; thus people with PKU (which can be diagnosed at birth) must be restricted to diets low in phenylalanine.
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore





