Carbonic acid, H 2 CO 3 , is commonly assumed to be an unstable compound that easily
Question:
Carbonic acid, H2CO3, 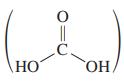 is commonly assumed to be an unstable compound that easily decomposes into a molecule of water and a molecule of carbon dioxide: H2CO3 → H2O + CO2↑. Indeed, the evidence of our own experience in opening a container of any carbonated beverage supports this apparently obvious notion. However, in 2000 it was discovered that this assumption is not quite correct: Carbonic acid is actually a perfectly stable, isolatable compound in the complete absence of water. Its decomposition, which is a decarboxylation reaction, is strongly catalyzed by water. It is exceedingly difficult to completely exclude water without the use of special techniques, which explains why carbonic acid has been such an elusive species to obtain in pure form.
is commonly assumed to be an unstable compound that easily decomposes into a molecule of water and a molecule of carbon dioxide: H2CO3 → H2O + CO2↑. Indeed, the evidence of our own experience in opening a container of any carbonated beverage supports this apparently obvious notion. However, in 2000 it was discovered that this assumption is not quite correct: Carbonic acid is actually a perfectly stable, isolatable compound in the complete absence of water. Its decomposition, which is a decarboxylation reaction, is strongly catalyzed by water. It is exceedingly difficult to completely exclude water without the use of special techniques, which explains why carbonic acid has been such an elusive species to obtain in pure form.
Based on the discussion of the mechanism of decarboxylations of 3-ketocarboxylic acids role for a molecule of water to play in catalyzing the decarboxylation of carbonic acid.
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore




