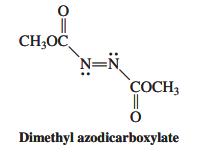
Dimethyl azodicarboxylate (see margin) takes part in the Diels-Alder reaction as a dienophile. Write the structure of
Question:
Dimethyl azodicarboxylate (see margin) takes part in the Diels-Alder reaction as a dienophile. Write the structure of the product of cycloaddition of this molecule with each of the following dienes.
(a) 1,3-Butadiene;
(b) trans,trans-2,4-hexadiene;
(c) 5,5-dimethoxycyclopentadiene;
(d) 1,2-dimethylenecyclohexane. Ignore the stereochemistry at nitrogen in the products (amines undergo rapid inversion)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





