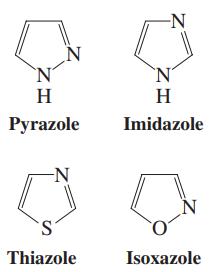
Each of the heterocyclopentadienes in the margin contains more than one heteroatom. For each one, identify the
Question:
Each of the heterocyclopentadienes in the margin contains more than one heteroatom. For each one, identify the orbitals occupied by all lone electron pairs on the heteroatoms and determine whether the molecule qualifies as aromatic. Are any of these heterocycles a stronger base than pyrrole?
Transcribed Image Text:
-N `N' H H Pyrazole Imidazole N S. Thiazole Isoxazole
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
Pyrazoleimidazolethiazole and isoxazole all are aromatic moleculesAll the above molecules has 4n2 e...View the full answer

Answered By

Alex Chacko
I am Alex Chacko, a second year integrated Msc Chemistry student at Institute for intensive research in basic science (IIRBS).I have also been working as a question and answer expert with chegg for the past two years.I have been answering difficult questions for the past 2 years and hence i assure you to provide the best quality answer.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Each of the following heterocycles includes one or more nitrogen atoms. Classify each nitrogen atom as strongly basic or weakly basic, according to the availability of its lone pair of electrons. (a)...
-
In the following acid-base reactions, 1. Determine which species are acting as electrophiles (acids) and which are acting as nucleophiles (bases). 2. Use the curved-arrow formalism to show the...
-
Pyrrole undergoes electrophilic aromatic substitution more readily than benzene, and mild reagents and conditions are sufficient. These reactions normally occur at the 2-osition rather than the...
-
The following is accounting information taken from Woodrail Company's adjusted trial balance for 2016: In addition, the following changes occurred in selected accounts during 2016: Required: Using...
-
Use the entire panel data set in AIRFARE.RAW for this exercise. The demand equation in a simultaneous equations unobserved effects model is Log(passenit) = it + 1 log(fareit) + ait + uit, where we...
-
Find the details about complex numbers and perform the basic arithmetic related to complex numbers. What do you expect when you perform mean, median, and sd on an array of complex numbers? Check the...
-
2. Prove that there is no continuous function whose Fourier coefficients satisfy lak(f)1 2: I/Vk for kEN.
-
Piccadilly Hospital has purchased new lab equipment for $200,000. The equipment is expected to last for three years and to provide cash inflows as follows: Year 1. . . . . . . . . . . . . . . . . . ....
-
Una de las principales diferencias entre empresas de servicios y fabricantes es que: opcin multiple Las empresas de servicios tienen materias primas, pero los fabricantes no. Las empresas de...
-
Start of Payroll Project 7-3a October 9, 20-- No. 1 The first payroll in October covered the two workweeks that ended on September 26 and October 3. This payroll transaction has been entered for you...
-
Rank the following compounds in increasing order of basicity: water, hydroxide, pyridine, pyrrole, ammonia.
-
Give the product of each of the following reactions. (a) (b) CH,NH2
-
Under what conditions should you use the two-way ANOVA F test to examine possible differences among the means of each factor in a factorial design?
-
Glowbright Company makes three types of long-burning scented candles. The models vary in terms of size and type of materials (fragrance, decorations, etc.). Unit information for Glowbright follows:...
-
EPI is considering outsourcing the production of the handheld control module used with some of its products. The company has received a bid from Control Freak Co. (CFC) to produce 10,000 units of the...
-
Take the diagnostic reasoning situation developed in Table 9.1 and 9.2 of the Dempster Shafer model of Section 9.2.3 and recast it as a Bayesian Belief network. Compare and contrast these two...
-
In your audit of the Whitestable Company's December 31, 1999 financial statements, you become aware of the following controls or procedures over investments, debt, and equity: a. On June 15, 1999,...
-
Christopher Rossi, CPA has been engaged by the Barrington Company, a nonpublicly held manufacturer of children's toys, to review Harrington's December 31, 1999 financial statements. Rossi accepts the...
-
Practice Program 5.4 asked you to define a Trivia class that contained strings representing a trivia question and answer to that question. Add an integer for the number of points that the question is...
-
Imagine a sound wave with a frequency of 1.10 kHz propagating with a speed of 330 m/s. Determine the phase difference in radians between any two points on the wave separated by 10.0 cm.
-
Anethole, C10H12O, a major constituent of the oil of anise, has the 1H NMR spectrum shown. On oxidation with Na2Cr2O7, Anethole yields p-methoxybenzoic acid. What is the structure of Anethole? Assign...
-
How would you synthesize Anethole (Problem 18.55) from phenol?
-
Aldehydes and ketones undergo acid-catalyzed reaction with alcohols to yield hemiacetals, compounds that have one alcohol-like oxygen and one ether-like oxygen bonded to the same carbon. Further...
-
ACC 2 0 2 Milestone One: Operational Costs Data Appendix You plan to open a small business for manufacturing pet collars, leashes, and harnesses. You have found a workshop space you can use for...
-
Problem 3 Progress Company acquired 6 0 % of Stall Corporation on 1 2 0 2 0 . Fair values of Stall's assets and liabilities approximated book values on that date. Progress uses the initial value...
-
Carla Vista Cart Inc. has the following information for 2026 : The rate of return on assets Carla Vista Cart Inc. is 81.06%17.27%30.58%14.00%

Study smarter with the SolutionInn App


