Formulate a mechanism for the reaction of acetyl chloride with 1-propanol shown on p. 932. Page 932
Question:
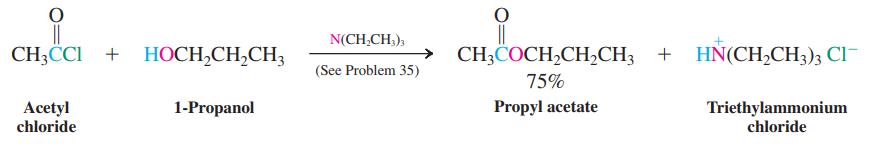
Formulate a mechanism for the reaction of acetyl chloride with 1-propanol shown on p. 932.
Page 932
Transcribed Image Text:
N(CH,CH,), CH3CCI + HOCH,CH,CH3 → CH;COCH,CH,CH3 + HN(CH,CH3); Cl (See Problem 35) 75% Propyl acetate Acetyl chloride 1-Propanol Triethylammonium chloride
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
The reactant alcohol have two unpaired electron in oxygen atom that ...View the full answer

Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Propose a mechanism for the reaction of acetyl chloride with phenylmagnesium bromide to give 1,1-diphenylethanol. OH (1) ether solvent CH3-C CI 2 (2) H 0 acetyl chloride phenylmagnesium bromide...
-
Formulate a mechanism for the reaction described in Problem 35(b). Problem 35(b) (b) NBS (1 equivalent), hv
-
Formulate a mechanism for the reaction of butanedioic (succinic) anhydride with methanol shown on p. 935. Data From Problem 935 CH,OH H; + Alcoholysis (ester formation) 83% Propanoic anhydride...
-
Fun Cosmetics Ltd. is a skincare product manufacturer based in New Zealand that produces the LoveSkin instant moisturizer for women. Because of the reliability and quality of its products, it has...
-
To the model of U.S.-China trade presented in Section 6.2, add a third country, called Colombia. Suppose that the Colombian economy has 90 million unskilled workers and 60 million skilled workers,...
-
What class do you use to work with random access files?
-
(Information Related to Various Bond Issues) Karen Austin Inc. has issued three types of debt on January 1, 2007, the start of the companys fiscal year. (a) $10 million, 10-year, 15% unsecured bonds,...
-
Compensation linked with profitability, waiting time, and quality measures. Mid-Atlantic Healthcare operates two medical groups, one in Philadelphia and one in Baltimore. The semi-annual bonus plan...
-
Which of the following is not considered to be an operating budget? A. Capital Budget B. Purchases Budget C. Cash Budget D. Sales Budget
-
On 1 January 2023, Crimmock Ltd (which prepares accounts to 31 December) enters into a four-year lease of office machinery. The company is required to make four lease payments of 30,000 and these...
-
Give the product(s) of each of the following reactions. (a) (b) (c) (d) (e) NH2 CH;CCI + 2
-
Give the product(s) of the reactions of acetic anhydride with each of the following reagents. Assume in all cases that the reagent is present in excess. (a) (CH 3 ) 2 CHOH (b) NH 3 (c) (d) LiAlH 4 ,...
-
Birthday problem. Suppose that people enter an empty room until a pair of people share a birthday. On average, how many people will have to enter before there is a match? Run experiments to estimate...
-
1) Special Relativity. Statement: Imagine this situation: Alice stands in New York City while Bob, aboard a plane departing from Boston, directly crosses over Alice at t=0. Disregard the vertical...
-
According to the College Board website, the scores on the math part of the SAT (SAT-M) in a certain year had a mean of 507 and a standard deviation of 111. Assume that SAT scores follow a normal...
-
Pay and incentive programs are being used both for knowledge workers and in non-knowledge worker occupations. In every industry, from restaurants to construction and low-tech manufacturing, companies...
-
Closet International invested in an equipment in 2019 with an initial cost of $598,000. It falls under asset class 8 with a CCA rate of 20%. The equipment was sold in 2021 for $260,000. Calculate the...
-
Question 4 (30 Marks) A 12-ply Kevlar/Epoxy composite beam with layup [0/90 / 0 1s is loaded in 3-point bending, as shown in Figure Q4. The beam has a length, L of 100mm, a width, b of 25mm and a...
-
Suppose bibliographic entries in Figure C 14.23 contain a mandatory key element, and that other documents can contain matching cite elements. Create an XSLT script that imitates the work of BibTEX....
-
The nitrogen atoms in N2 participate in multiple bonding, whereas those in hydrazine, N2H4, do not. (a) Draw Lewis structures for both molecules. (b) What is the hybridization of the nitrogen atoms...
-
Draw all the stereo isomers of 2-bromo-3-chlorobutane and indicate whether they are enantiomers or diastereomers.
-
Label each chirality center in these compounds with an asterisk and calculate the maximum number of stereo isomers for each. CI a) b) d) c)
-
Draw all of the stereo isomers for 2, 3-dichlorobutane. Indicate which rotate plane-polarized light and which are meso.
-
Hiorusital maiyus of income statement if required, round percentages to one dectmat place. Mackiln lne: Cemparative Income statement Fer the Years tnded December 31, 20rz and zors...
-
1. What is the primary benefit of just-in-time (JIT) systems compared with traditional materials requirement planning (MRP) systems? a. Replacement of a "demand pull" manufacturing strategy with...
-
b. If the coupon code provided a 20% discount, what is the maximum abandon rate that will still provide a better net profit than offering no discount?

Study smarter with the SolutionInn App


