Give the product(s) of the reactions of acetic anhydride with each of the following reagents. Assume in
Question:
Give the product(s) of the reactions of acetic anhydride with each of the following reagents. Assume in all cases that the reagent is present in excess.
(a) (CH3)2CHOH
(b) NH3
(c)
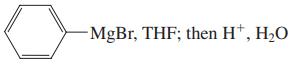
(d) LiAlH4, (CH3CH2)2O; then H+, H2O
Transcribed Image Text:
MgBr, THF; then H*, H2O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 52% (17 reviews)
a Nucleophilic substitution reaction two moles of easters are formed b Tetr...View the full answer

Answered By

Archana Prasad
Hi! My name is Archana Prasad. I did my graduation in Biotechnology in year 2011 from Patna University. After that I completed my Master degree from Patna Science College, Patna University in BIOCHEMISTRY in year 2013. During the session I participated in various seminars and internships at reputed platform such as Sudha Diary, Patna and perform well there.
As per my tutoring experience is concern I have expertise of 4 years at reputed coaching institutions in Patna, Bihar. I have also experience of solving questions on various online platform. I have worked with PhotoStudy as well as Studypool.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Give the product(s) of each of the following reactions. (a) (b) (c) (d) (e) NH2 CH;CCI + 2
-
Give the product(s) of each of the following reactions, ignoring stereoisomers: (a) (b) (c) (d) (e) (f) hv CH CH,C CHCHNBS peroxide CH h CH3CH-CHCH.CH-CH3 Br2 + hv + Cl CH2Cl2 +Cl2 CH3 + Cl2
-
Give the product(s) obtained from the reaction of each of the following compounds with Br2/FeBr3: a. b. c. d. O-C COCH3 NO2 CH3O
-
Q1. a)Analyse Spencers experience in terms of consumer behavior framework in your notes. What variables and processes can you identify that appear to work in this situation? Does this situation...
-
Consider an economy in which two factors of production, labor and capital; produce two goods, capital-intensive pharmaceuticals and labor-intensive clothing. Suppose that both factors of production...
-
How do you display a frame on the screen?
-
(Entries for Bond Transactions) On January 1, 2007, Aumont Company sold 12% bonds having a maturity value of $500,000 for $537,907.37, which provides the bondholders with a 10% yield. The bonds are...
-
Marx Industries had the following transactions. 1. Borrowed $5,000 from the bank by signing a note. 2. Paid $3,100 cash for a computer. 3. Purchased $850 of supplies on account. Instructions (a)...
-
Sterling, Inc., is an export-import firm. On June 1, Sterling purchased goods from a British company costing 20,000. Payment is due in pounds on September 1. The spot rate on June 1 was $1.29 per...
-
Heavy Duty, a manufacturer of hydraulic tipper trailers for the mining business and its competitor Bullmaster are located in Folfanga. Folfanga does not charge corporate taxes. In the local stock...
-
Formulate a mechanism for the reaction of acetyl chloride with 1-propanol shown on p. 932. Page 932 N(CH,CH,), CH3CCI + HOCH,CH,CH3 CH;COCH,CH,CH3 + HN(CH,CH3); Cl (See Problem 35) 75% Propyl...
-
Give the product(s) of the reaction of butanedioic (succinic) anhydride with each of the reagents in Problem 36. Data From Problem 36 (a) (CH 3 ) 2 CHOH (b) NH 3 (c) (d) LiAlH 4 , (CH3CH 2 ) 2 O;...
-
Derive Equation 11.4.2 from Equation 11.4.1. i-1 i-1
-
Scenario: You have been working in a community service sector for two years. However, you always find evaluating your own performance challenging. Your Supervisor has also identified that you do not...
-
Tristan Walker of Walker & Company says, "We are only going to design, develop, and test products and services uniquely tailored to our community's needs. I get it. I'm a part of the community we are...
-
Ace Cosmetics Corporation purchased land adjacent to its plant to improve access for trucks making deliveries. Expenditures incurred in purchasing the land were as follows: purchase price, $55,000;...
-
7. At this point you now know information about both the horizontal and the vertical components of the projectile's velocity. In the space below, draw a diagram of the vector components of Vx and...
-
Complete autonomy in how you demonstrate the following criteria. In this module, we talked more about leadership. We discussed the differences between leadership theory which is a well-substantiated...
-
If you were writing a two-pass compiler, why might you choose a high level IF as the link between the front end and the back end? Why might you choose a medium-level IF?
-
By referring to Figure 13.18, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 oC: (a) KClO3, (b) Pb(NO3)2, (c) Ce2(SO4)3.
-
Draw all of the stereo isomers of 1, 2-dimethylcyclopropane. Explain which rotate plane-polarized light.
-
Explain whether these compounds rotate plane-polarized light: H a) HC-CCH, HC Br c) Br J. s CI b) Cl CI d) HC -CH3
-
Draw Fischer projections for these compounds? CHOH a) H-C-CI CH3 COH b) HC-OH A CH3 c) H CHCH CH CH3
-
Securities A and B have betas of 1.15 and 1.41, respectively. The standard deviation of returns is 22% for security A and 12% for security B. Which security should earn higher expected return? A)...
-
You plan to invest in Stock X, Stock Y, or some combination of the two. The expected return for X is 10% and x= 5%. The expected return for Y is 12% and y = 6%. The correlation coefficient, xy, is...
-
Edgar SEC 10k (2/31/17) is located http://www.sec.gov/edgar/searchedgar/companysearch.html. Using Masco Corporation (SIC 2430), 1. I there a reliance on a small number of large customers? If, yes who...

Study smarter with the SolutionInn App


