Friedel-Crafts acylations are best carried out with acyl halides, but other carboxylic acid derivatives undergo this process,
Question:
Friedel-Crafts acylations are best carried out with acyl halides, but other carboxylic acid derivatives undergo this process, too, such as carboxylic anhydrides or esters. These reagents may have some drawbacks, however, the subject of this problem. Before you start, discuss as a group the mechanisms for forming acylium ions from acyl halides and carboxylic anhydrides. Then divide your group in two and analyze the outcome of the following two reactions. Use the NMR spectral data given to confirm your product assignments.
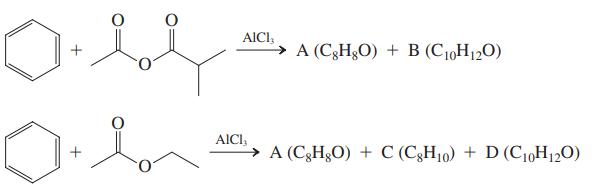
Compound A: 1H NMR: δ 2.60 (s, 3H), 7.40 – 7.50 (m, 2H), 7.50 – 7.60 (m, 1H), 7.90 – 8.00 (m, 2H).
Compound B: 1H NMR: δ 2.22 (d, 6H), 3.55 (sep, 1H), 7.40 – 7.50 (m, 2H), 7.50 – 7.60 (m, 1H), 7.90 – 8.00 (m, 2H).
Compound C: 1H NMR: δ 1.20 (t, 3H), 2.64 (q, 2H), 7.10 – 7.30 (m, 5H).
Compound D: 1H NMR: δ 1.25 (t, 3H), 2.57 (s, 3H), 2.70 (q, 2H), 7.20 (d, 2H), 7.70 (d, 2H).
Reconvene to share your solutions. Then specifi cally address the nature of the complications ensuing when using the reagents shown. Finally, all together, consider the following reaction sequence. Again, use a mechanistic approach to arrive at the structures of the products.
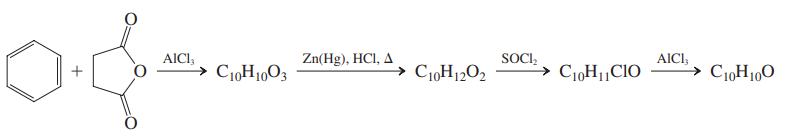
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore





