Give the expected major product(s) of each of the following electrophilic substitution reactions. N(CH3)2 CI CCH,CH, CH.CCI,
Question:
Give the expected major product(s) of each of the following electrophilic substitution reactions.
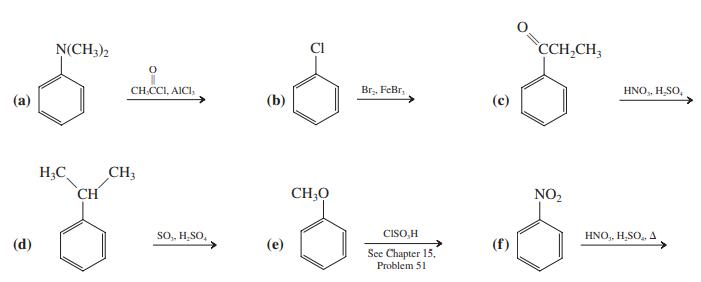
Transcribed Image Text:
N(CH3)2 CI CCH,CH, CH.CCI, AICI, Br,, FeBr, HNO,. H,SO, (a) (b) H;C CH; CH CH;0 NO2 SO,, H,SO, CISO,H HNO,, H,SO,, A (d) (e) (f) See Chapter 15, Problem 51
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 68% (16 reviews)
Diagramatic representation of major product of the given reaction is shown in attached image a the g...View the full answer

Answered By

User l_359487
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Give the expected major product(s) of each of the following reactions. 1. Hg(OCH,),. CH,OH 2. NaBH, CH,OH CH=CH2 CH3 (b) H2C=C 1. CH,COOH, CH,CI, 2. H', . Conc. HI (a) CH3OCH,CH,CH=CH2 CH,OH 1....
-
Give the expected major product(s) of each of the following reactions. NO2 COOH CH,CH; Cl, FeCI, So,, H,SO, HNO,, H,SO, () (b) CH3 NHCH, SO,H CH3 Brs, FeBr, Br,, FeBr, SO, H;SO, (d) (e) CH CCH3 NO2...
-
Give the expected major product of each of the following reactions. (a) (b) (c) (d) CH CH,CH,OH COnC HI Conc. HBr (CH,)CHCH.CH.OH- Conc. HI OH CH,CH2,COH COn HCT
-
Roberts Originals Co. (ROC) provides new and unique cases and otherassignments to professors each semester to ensure that students will not be able to find the solutions published online. Due to the...
-
Violet complained to the police as follows: After a domestic dispute, her boyfriend took away the keys to her car, forced her into his car, threatened her, and slapped her. As the boyfriend slowed...
-
What is the purpose of an abstract method?
-
1 Do your studies and related activities on your course satisfy needs identified by Maslow?
-
Louie Anderson works in the production department of Southwest Plasticworks as a machine operator. Louie, a long-time employee of Southwest, is paid on an hourly basis at a rate of $ 20 per hour....
-
You are self-employed. If your business earns $100,000 in net income in 2014 and pays 15.3% of that for self-employment taxes (Social Security, Medicare, and Medicaid), then how much does your...
-
The U.S. has expected inflation of 2%, while Country A, Country B, and Country C have expected inflation of 7%. Country A engages in much international trade with the U.S. The products that are...
-
Draw appropriate resonance forms to explain the activating ortho, para-directing character of the phenyl substituent in biphenyl Biphenyl
-
Reaction review. Without consulting the Reaction Road Map, suggest a combination of reagent and monosubstituted benzene that would give each of the following compounds. Refer to Table 16-2 for...
-
Expand 1/4 - 2x in ascending powers of x, up to and including the term in x 2 .
-
Due to a crash at a railroad crossing, an overpass is to be constructed on an existing level highway. the existing highway has a design speed of 50 mi/h. The overpass structure is to be level,...
-
Finding Bone Density Scores. In Exercises 37-40 assume that a randomly selected subject is given a bone density test. Bone density test scores are normally distributed with a mean of 0 and a standard...
-
Use the Comparison Theorem to determine whether the integral is convergent or divergent. L da
-
Problem 3 (2 scenarios) Scenario 1: Rocky Inc hired a new intern from CSU to help with year-end inventory. The intern computed the inventory counts at the end of 2020 and 2021. However, the intern's...
-
A CM reactor receives influent containing 10.0 mg/L of tracer for 2 h. Then tracer addition is terminated but the flow remains steady. The volume of the reactor is 10 L and the flow rate is 2 L / h....
-
Newly formed S&J Iron Corporation has 50,000 shares of $10 par common stock authorized. On March 1, Year 1, S&J Iron issued 6,000 shares of the stock for $16 per share. On May 2, the company issued...
-
A consumer magazine is evaluating five brands of trash compactors for their effectiveness in reducing the volume of typical household products that are discarded. In the experiment, each block...
-
The limiting molar conductivities of NaI, NaCH3CO" and Mg(CH3C02)2 are 12.69 mS m2 mol-1, 9.10 mS m2 mol-1, and 18.78 mS m2 mol-1, respectively (all at 25C). What is the limiting molar conductivity...
-
At 25C the molar ionic conductivities of F3, er, and Bc are 5.54 mS m2 mol-1, 7.635 mS m2 mol-1, and 7.81 mS m2 mol-1, respectively. What are their mobilities?
-
The mobility of a CH1COi ion in aqueous solution at 25C is 4.24 x 10-8 m2 S-1 V-1 Calculate its diffusion coefficient in water at 25C.
-
Suppose you deposit $1,071.00 into an account 4.00 years from today. Exactly 13.00 years from today the account is worth $1,477.00. What was the account's interest rate? Assume the real rate of...
-
What are the primary factors that contribute to fraudulent activity
-
- . . / , , 2 , 5 % . . - . . - . . Q )

Study smarter with the SolutionInn App


