Give the expected major product(s) of each of the following reactions. NO2 COOH CH,CH; Cl, FeCI, So,,
Question:
Give the expected major product(s) of each of the following reactions.
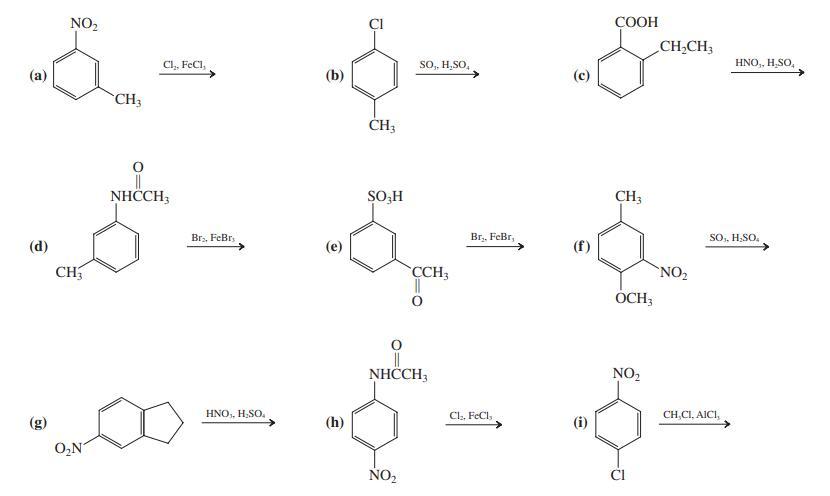
Transcribed Image Text:
NO2 COOH CH,CH; Cl, FeCI, So,, H,SO, HNO,, H,SO, (а) (b) CH3 NHCH, SO,H CH3 Brs, FeBr, Br,, FeBr, SO, H;SO, (d) (e) CH CCH3 NO2 OCH; NHČCH, NO, HNO,, H;SO, Cl., FeCl, CH,CI, AICI, (g) (h) (i) O,N ÑO2 CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 87% (8 reviews)
Expected major produ...View the full answer

Answered By

Vivek Chaudhary
Hi! I am vivek.My greatest passion in life is teaching. I was born and raised in india, and experienced great success at school and at university due to amazing and unforgettable teachers. This is the foundation of my commitment to helping out my students, whatever their abilities may be. Currently, I am studying a masters degree specializing in chemistry, rounding out my undergraduate background in chemistry (Honors) I have been tutoring and teaching for 2 years in various settings – tutoring small and large groups.
So, if you stuck in any problem you can ask.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Give the expected major product of each of the following reactions. PCC is the abbreviation for pyridinium chlorochromate (Section 8-6). (a) (b) (c) (d) (e) CH CH CH,OH NeCrO HSO, HO PCC, CH,CI (CH3)...
-
Give the expected major product of each of the following reaction sequences. PCC refers to pyridinium chlorochromate. (a) (b) (c) 1. CrO, H,SO., HO 2. CH.CHMgBr, (CH,CH, 3. H, H,O CHs) CHOH 1. OH,...
-
Give the expected major product of each of the following reactions. (a) (b) (c) (d) CH CH,CH,OH COnC HI Conc. HBr (CH,)CHCH.CH.OH- Conc. HI OH CH,CH2,COH COn HCT
-
The income statement for Performance Limited is given below along with some supplementary information. Performance Limited Income Statement for the year ended December 30, 2020 (in $000s) Division A...
-
In this criminal case, defendant was found guilty of aggravated kidnapping. The crime arose out of a situation involving a domestic dispute. After a fight, defendant abducted his girl friend, beat...
-
True or False: If a subclass constructor does not explicitly call a superclass constructor, Java will not call any of the superclasss constructors.
-
1 How did the balance between the two sets of expectations have on your behaviour?
-
Compare the advantages and disadvantages of the two primary methods used to allocate joint cost to joint products.
-
Which of the following statement(s) about index funds is false: the EMH provides a theoretical justification for them they have high R2 s they outperform most actively managed funds over a long...
-
A U.S. company estimated that, in the first two months of 2016, its export sales to a Swiss company would generate 400,000 francs. On December 1, 2020, in an effort to protect against the weakening...
-
Reaction review. The preparation of the following compounds requires more than one step. As in Problem 41, suggest a monosubstituted benzene as starting material and the reagents for all the steps...
-
(a) When a mixture containing one mole each of the three dimethylbenzenes (o-, m-, and p-xylene) is treated with one mole of chlorine in the presence of a Lewis acid catalyst, one of the three...
-
The comparative balance sheet of Charles Inc. for December 31, 2014 and 2013, is shown as follows: Additional data obtained from an examination of the accounts in the ledger for 2014 are as follows:...
-
A four-lane urban freeway (two lanes in each direction) is located on rolling terrain and has 12-ft lanes, no lateral obstructions within 6 ft of the pavement edges, and an interchange every 2 miles....
-
In January, 1993, there were about 1,313,000 internet hosts. During the next five years, the number of hosts increased by about 100% per year. a. Write a model giving the number h (in millions) of...
-
Allison, Inc., produces two products, X and Y, in a single joint process. Last month the joint costs were P75,000 when 10,000 units of Product X and 15,000 units of Product Y were produced....
-
8+0.5 = 4. Consider a system with a lead compensator Ge(s) = +0.13 followed by a plant G(s) = 10 Determine a value for a gain K on the error signal such that the phase margin s(s+1) of the open-loop...
-
Standard Normal Distribution. In Exercises 17-36, assume that a randomly selected subject is given a bone density test. Those test scores are normally distributed with a mean of 0 and a standard...
-
Obtain the Target Corporations annual report for its 2018 fiscal year (year ended February 2, 2019) at http://investors.target.com using the instructions in Appendix A, and use it to answer the...
-
On March 31, 2018, Gardner Corporation received authorization to issue $30,000 of 9 percent, 30-year bonds payable. The bonds pay interest on March 31 and September 30. The entire issue was dated...
-
A Knudsen cell was used to determine the vapour pressure of germanium at 1000C. During an interval of7200 s the mass loss through a hole of radius 0.50 mm amounted to 43 ug, what is the vapour...
-
An atomic beam is designed to function with (a) Cadmium, (b) Mercury. The source is an oven maintained at 380 K, there being a small slit of dimensions 1.0 cm x 1.0 x 10-3 cm. The vapour pressure of...
-
The resistances of a series of aqueous NaCI solutions, formed by successive dilution of a sample, were measured in a cell with cell constant (the constant C in the relation K= C/R) equal to 0.2063...
-
Complete this uestion by entering your answers in the tabs below. Calculate the year-end adjusted balances of Supplies and Supplies Expense. Beaver Construction purchases new equipment for $31,200...
-
Dorsey Company manufactures three products from a common input in a joint processing operation. Joint processing costs up to the split - off point total $ 3 0 5 , 0 0 0 per quarter. For financial...
-
Arkell Companys December 31, 2020 equity section of the balance sheet included the following: Common shares, 60,000 shares issued and outstanding.. $1,500,000 Retained earnings 1,300,000 On February...

Study smarter with the SolutionInn App


