Give the product of each of the following reactions. (a) (b) (c) (d) (e) CH 1. ,
Question:
Give the product of each of the following reactions.
(a)
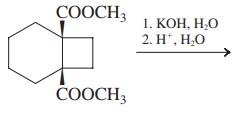
(b)
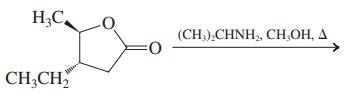
(c)
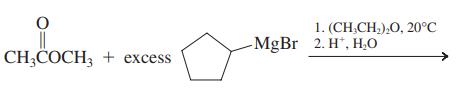
(d)
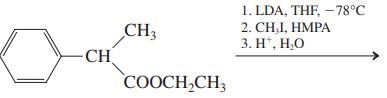
(e)
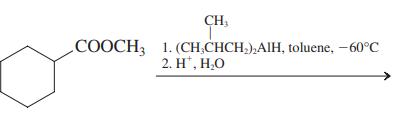
Transcribed Image Text:
СООCH 1. КОН, Н.О 2. H', Н.О СООCH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
a...View the full answer

Answered By

MANDWEEP BHUMIJ
I did my masters in Tezpur University with specialization in Organic chemistry. I cleared CSIR NET-JRF with all India ranking 74. I am currently working as Junior Research Fellowship in Central drug research institute Lucknow. I have some private tutoring experiences in multiple subjects.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Give the product of each of the following reactions: (a) (b) (c) (d) CH3 CHCH3 Na2r207.H CH2CH3 Na,Cr0H CH3 1. NBS/A/peroxide 2. CH30 CH3 1. NBS/A /peroxide 3. H2/Ni
-
Give the product of each of the following reactions: a. b. c. d. OHH, . CHCHCH,N12 catalytic Zo outo 0 auda CH3CCI + 2 1. HCI, NaNO2 22. H20, Cu20, Cu(NO32 catalytic
-
Give the product of each of the following reactions: a. b. c. d. CH CH3 A CH2CH3 CH2CH 2CH3 -A CH2CH3 h CH2CH3 CH2CH3 CH2CHs
-
Your restaurant has five menu choices for lunch. How many ways can you order them on your menu? A 0.0749 B 0.0747 120
-
Consider the following thought experiment regarding foreign direct investment and specific factors. (a) In the pure specific-factors model in the text, suppose that a wave of foreign direct...
-
What is the preferred way of creating a Border object?
-
(Amortization SchedulesStraight-line) Devon Harris Company sells 10% bonds having a maturity value of $2,000,000 for $1,855,816. The bonds are dated January 1, 2007, and mature January 1, 2012....
-
In a hypothetical Linux system, each virtual address is 32 bits with a 10-bit page size, 256-entry page table, 32l-entray page middle directory, and 256-entry page directory. Using the Linux model...
-
What is the market-to-book ratio for 2017? 3.00 times 3.51 times 3.89 times 5.12 times
-
Procter & Gamble has been the leading soap manufacturer in the United States since 1879, when it introduced Ivory soap. However, late in 1991, its major rival, Lever Bros. (Unilever), overtook it by...
-
Reaction review. Suggest reagents to convert each of the following starting materials into the indicated product: (a) hexanoyl chloride into acetic hexanoic anhydride; (b) methyl hexanoate into...
-
For each of the naturally occurring lactones below, draw the structure of the compound that would result from hydrolysis using aqueous base. (a) (b) (c) Sedanenolide, major contributor to the flavor...
-
Name and interpret the two sources of variation in the one-way analysis of variance.
-
Multi-national management in a global economy requires a variety of hard and soft skills. This assignment is meant to enhance the understanding of multi-national situations locally or globally, and...
-
Which one of the following is not a part of the Deployment phase of a machine learning development project? Explain what phase(s) address this issue, and why then? Training end users to incorporate...
-
Assist with the following discussion: Topic Discussion #1B: The first half of the term is devoted to leaders preparing themselves for leadership. Peter Senge and his coauthors discuss in The Dawn...
-
You are managing an employee who is not a self-starter, and thus you need to devise a plan to effectively lead this employee. Draft a one page (Times New Roman 12) single space response (plus title...
-
Ontario's minister of training, colleges and universities defended changes to post-secondary education on Monday, saying recently announced decisions are all about the making the system more...
-
Suppose that k (the number of temporary registers) in Figure 15.6 is 4 (this is an artificially small number for modern machines). Give an example of an expression that will lead to register spilling...
-
What is the maximum volume of 0.25 M sodium hypochlorite solution (NaOCl, laundry bleach) that can be prepared by dilution of 1.00 L of 0.80 M NaOCl?
-
Identify these pairs of compounds as identical, structural isomers, enantiomers, or diastereomers: CH3 CH3 H3 CH Br - - - - -Cl - b) Br- a) - - CI - - CH3 CH CH3 CH3 CH3 CH3 CH CI Br - - CH3...
-
(a) A solution of 0.2g/mL of a compound in a 1 dm cell rotates plane-polarized light + 13.3 o at the sodium D line. What is the specific rotation of this compound? (b) What is the rotation caused by...
-
Describe how this amine could be resolved by using this carboxylic acid? CH3 CNH, H HC H CI C COH
-
Calculate the monthly finance charge for the credit card transaction. Assume that it takes 10 days for a payment to be received and recorded, and that the month is 30 days long. (Round your answers...
-
Homework 1 0 - Chapter 2 1 i 1 0 points Use the information below to answer the questions that follow. \ table [ [ , U . S . $ EQUIVALENT,CURRENCY PER U . S . $ ] , [ U . K . Pound ( ) , 1 . 5 8 3 8...
-
Pooler purchased 6 1 % of Scenic's outstanding common stock for $ 6 4 6 , 4 1 9 on January 1 , 2 0 X 6 , when the book value of Scenic's net assets was equal to $ 3 6 5 , 0 0 0 . The non -...

Study smarter with the SolutionInn App


