Give the products of each of the following reactions. (a) (CH 3 ) 2 CHCH 2 CO
Question:
Give the products of each of the following reactions.
(a) (CH3)2CHCH2CO2H + SOCl2 →
(b) (CH3)2CHCH2CO2H + CH3COBr →
(c)
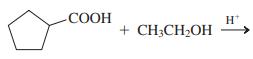
(d)
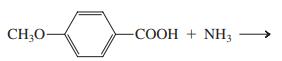
(e) Product of (d), heated strongly
(f) Phthalic acid, heated strongly
Transcribed Image Text:
СООН H + CH;CH2OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a CHCHCHCOOH SoCl CHCHCHCCl SO HCl Acid react with thionyl chloride to ...View the full answer

Answered By

Bharat sharma
• Strong theoretical and practical knowledge of chemistry
• In-depth knowledge of chemistry and ability to teach students
• Ability to develop and execute lessons plans
• Skilled in a variety style of teaching methods and techniques
• Experience of conducting research and documenting results
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Give the products of each of the following reactions: a. b. c. d. CH,-CH-CH-CH2 + CH3C-C= C-CCH, CH CH CH3 CHa CH3C CH-CH-CCH3
-
Give the products of each of the following reactions: a. b. c. d. e. f. g. h. HCI CH CH2CH CHCH2OH excess catalytic CCH CH3 NH2NH2 CH2CH3 NH2NH 1. NaBH4 0 HCI excess CH,CH,CH,COCH CHs 2. H3o 1. LIAIH...
-
Give the products of each of the following reactions: a. b. c. d. e. f. g. h. i. j. HCI CH2CH3 1. CH3CH2MgBr 1. CH3CH2MgBr excesS CH,CH,COCH 2. H20 ot.cum 1. LiAIH4 NO 2. H20 catalytic Ht + CH...
-
Write a memo to an employee whose work and behavior have been unsatisfactory. (You are the department manager.) Try to motivate the employee to improve his performance. Be brief, direct, honestand as...
-
Consider the model of Section 13.2. Suppose that is initially positive but increases due to tightening emissions regulation (choose either the case < * or > * and stick to it). Trace through the...
-
Describe the changes that would need to be made to the binary search tree implementation given in the book to allow it to be used to support an ordered dictionary, where we allow for different...
-
Honor de Balzac Inc. has been producing quality childrens apparel for more than 25 years. The companys fiscal year runs from April 1 to March 31. The following information relates to the obligations...
-
Decker Company has five products in its inventory. Information about the December 31, 2018, inventory follows. The cost to sell for each product consists of a 15 percent sales commission. Required:...
-
What is spot-futures parity? Explain
-
6. 7. 8. The lengths of various links of a mechanism, as shown in Fig. 6.32, are: OA = 0.3 m ; AB= 1 m; CD=0.8 m; and A C= CB. Determine, for the given configuration, the velocity of the slider D if...
-
(a) An unknown compound A has the formula C 7 H 12 O 2 and infrared spectrum A (p. 917). To which class does this compound belong? (b) Use the other spectra (NMR-B, p. 917, and F, p. 919; IR-D, E,...
-
When 1,4- and 1,5-dicarboxylic acids, such as butanedioic (succinic) acid (Section 19-8), are treated with SOCl2 or PBr3 in attempted preparations of the diacyl halides, the corresponding cyclic...
-
You want to estimate for the population of IQ scores of statistics professors. Find the minimum sample size needed to be 95% confident that the sample standard deviation s is within 1% of . Is this...
-
Differentiate. G(x) = (2x+3) (9x+ (x) G'(x)=
-
Consider the following C functions and assembly code: int fun4 (int *ap, int *bp) ( int a = *ap; int bbp; return a+b; }) pushl ebp movl esp, ebp int fun5 (int *ap, int *bp) { int bbp; *bp + *ap;...
-
The position of a particle moving along the x-axis is given by x(t) = = 4.2 2.5t m. (Assume t is in seconds.) (a) At what time (in s) does the particle cross the origin? 1.68 S (b) What is the...
-
2. Boxes A and B are being pulled to the right by a rope attached to box B. Box A sits on top of box B, and both boxes accelerate together to the right at a rate of 1.75 m/s. The masses and...
-
You bought a 15-kilogram sack of unshelled peanuts for your restaurant. You weigh the sack three times on a balance, with the following results: Trial Mass (kg) 1 15.02 2 15.49 3 15.91 The results...
-
The mechanism used in Figure 13.13 to make scheduler code reentrant employs a single OS-provided lock for all the scheduling data structures of the application. Among other things, this mechanism...
-
Show, if u(x, y) and v(x, y) are harmonic functions, that u + v must be a harmonic function but that uv need not be a harmonic function. Is e"e" a harmonic function?
-
Show a free energy versus reaction progress diagram for the following reaction: HCI+ NH3 CI + NH4
-
Which species is a stronger acid? a) HS or HCI + b) PH or NH c) CHCH3 or HS
-
Which anion is the stronger base? a) HO or HS b) CH3NH or CH0
-
Becton Labs, Incorporated, produces various chemical compounds for industrial use. One compound, called Fludex, is prepared using Required: For direct materials: a . Compute the price and quantity...
-
We sell an old asset today for $5 million. The book value of the asset is $7 million. Do we pay taxes or do we get tax credit? ______ By how much? _____
-
RiverRocks (whose WACC is 12.7%) is considering an acquisition of Raft Adventures (whose WACC is 14.1%). The purchase will cost $102.8 million and will generate cash flows that start at $14.1 million...

Study smarter with the SolutionInn App


