Give the product(s) of reaction of hexanal with each of the following reagents. (a) HOCH 2 CH
Question:
Give the product(s) of reaction of hexanal with each of the following reagents.
(a) HOCH2CH2OH, H+
(b) LiAlH4, then H+, H2O
(c) NH2OH, H+
(d) NH2NH2, KOH, heat
(e) (CH3)2CHCH2CH=P(C6H5)3
(f)
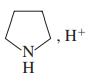
(g) Ag+, NH3, H2O
(h) CrO3, H2SO4, H2O
(i) HCN
Transcribed Image Text:
H+ N' H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
Give Products of react...View the full answer

Answered By

Samee Ullah
Algebra, Linear algebra, calculus, accounting, marketing, statistics, programming, real estate, writing, human resource management, business communication, Engineering: civil, chemical, electrical, mechanical, aerospace, building
Linguistics: sociolinguistics, applied linguistics, music, social sciences, biology, chemistry: all types, Thermodynamics, mechanics, modern physics, quantum physics, metaphysics, biology.
Feel free to contact us for all these subjects,; for quality, and best responses. Thankyou
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Give the product(s) of the following reaction sequences. 1. , * 2. 1. , * 2. (CH),CH.CI 3. ', . CH,Br 3. H., . () CH,CO (b) CH2CHO
-
Give the product(s) of each of the following reactions, ignoring stereoisomers: (a) (b) (c) (d) (e) (f) hv CH CH,C CHCHNBS peroxide CH h CH3CH-CHCH.CH-CH3 Br2 + hv + Cl CH2Cl2 +Cl2 CH3 + Cl2
-
Predict the product(s) of the following reaction, indicating stereochemistry wherenecessary: Br CH3 H20 Ethanol
-
A limited partnership: Multiple Choice has an unlimited life. can opt to be taxed as a corporation. terminates at the death of any one limited partner. has at least one partner who has unlimited...
-
Again, consider the East and West economy, in its first version of Question 4 with equal money supplies in the two countries. Suppose that at date 0 a technological breakthrough is announced that...
-
In general, what operations does the main application class perform?
-
1 6 Outline a model of the tactics that people use to influence others, including the use of cooperative networks
-
Anasazi Earth Clothing is a retail store specializing in womens clothing. The store has established a liberal return policy for the holiday season in order to encourage gift purchases. Any item...
-
financial position of Zomato and analyse the financial statement of the company
-
A spreadsheet containing financial statements for Mens Wearhouse, Inc., for fiscal years 20062010 is available for download from McGraw-Hills Connect or your course instructor. MEN'S WEARHOUSE INC....
-
Three isomeric ketones with the molecular formula C 7 H 14 O are converted into heptane by Clemmensen reduction. Compound A gives a single product upon Baeyer-Villiger oxidation; compound B gives two...
-
Give the product(s) of reaction of cycloheptanone with each of the reagents in Problem 52. Data From Problem 52 (a) HOCH 2 CH 2 OH, H + (b) LiAlH 4 , then H + , H 2 O (c) NH 2 OH, H + (d) NH 2 NH 2 ,...
-
Using the information in Problem 7B, prepare compound closing entries for Ucore Sales. In Problem 7B Ucore Sales Work Sheet For the Year Ended December 31, 2014 Balance Sheet & Statement of Changes...
-
Pacifico Company, a US-based importer of beer and wine, purchased 1,800 cases of Oktoberfest-style beer from a German supplier for 522,000 euros. Relevant U.S. dollar exchange rates for the euro are...
-
Complete the "Leadership Vision Questionnaire" in Chapter 7 (p176). Reflect on your results and complete the following prompts: Share the results from your questionnaire. Be sure to include the final...
-
1. Prepare el Presupuesto Operacional hasta completar el COGS (70 puntos) La empresa ACCO 295 tiene una venta proyectada de $450,000 Cada unidad se vende $450 Su inventario inicial 300 (costo $125)...
-
Continuing Case 65. Retirement Income Forecast Jamie Lee and Ross, now 57 and still very active, have plenty of time on their hands now that the triplets are away at college. They both realized that...
-
The partnership of Frick, Wilson, and Clarke has elected to cease all operations and liquidate its business property. A balance sheet drawn up at this time shows the following account balances: Cash...
-
The December 31, Year 4, balance sheet for Deen Company showed total stockholders equity of $156,000. Total stockholders equity increased by $65,000 between December 31, Year 4, and December 31, Year...
-
(8%) Problem 6: A student attaches a f= 3.5 kHz oscillator to one end of a metal rail of length L = 25 m. The student turns on the oscillator and uses a piezoelectric gauge at the other end to...
-
Benzene and hexane are both liquids at room temperature. Do you expect benzene and hexane to be miscible? Do you expect benzene and water to be miscible? Explain. Hexane 0 Benzene
-
One of these isomers is miscible with water, and the other is nearly insoluble. Explain. CHCHCHCOH CH,COCHCH3
-
Because of two hydrogen bonds, carboxylic acids show a very strong attractive force between two molecules that persists even in the gas phase. Show this hydrogen bonding between two carboxylic acid...
-
Which of the following is an item in a mortgage? Listing agreement Lease Deed of Trust Purchase agreement Please specify the correct answer
-
1.Why did Novo believe that its cost of capital was too high compared to its competitors? Why did Novos relatively high cost of capital create a competitive disadvantage? 2.What is meant by a...
-
On January 1, 2017, Sage Hill Inc. borrowed and receive $310,000 from a major customer, Sheffield Corp. The debt is evidenced by a zero-interest-bearing note due in 4 years. Sage Hill, as...

Study smarter with the SolutionInn App


