One of these isomers is miscible with water, and the other is nearly insoluble. Explain. CHCHCHCOH CH,COCHCH3
Question:
One of these isomers is miscible with water, and the other is nearly insoluble. Explain.
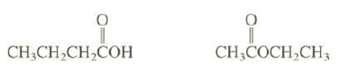
Transcribed Image Text:
CH₂CH₂CH₂COH CH,COCH₂CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (15 reviews)
The carboxylic acid is miscible with water be...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
There are two stereoisomers of 1-tert-butyl-4-chlorocyclohexane. One of these isomers reacts with sodium ethoxide in an E2 reaction that is 500 times faster than the reaction of the other isomer....
-
Water and glycerol, CH2(OH)CH(OH)CH2OH, are miscible in all proportions. What does this mean? How do the OH groups of the alcohol molecule contribute to this miscibility?
-
Explain why water forms nearly spherical droplets on the surface of a freshly waxed car. Why doesn't water bead on a clean windshield?
-
An investor has $60,000 to invest in a $280,000 property. He can obtain either a $220,000 loan at 9.5 percent for 20 years or a $180,000 loan at 9 percent for 20 years and a second mortgage for...
-
Assume that you have just been employed at Southern Coast Wealth Foods as an assistant and you are concerned with the high level of reliance on PCI to evaluate and compare the performance of the...
-
Lujie Xie is the controller of Lincoln Corporation and is responsible for the preparation of the year-end financial statements on December 31, 2017. Lincoln prepares financial statements in...
-
In each of the following cases, use the sample size formula to determine a sample size large enough to avoid constructing a p-chart with a negative lower control limit. a. p0 = .01 b. p0 = .05 c. p0...
-
Items from the 2013 income statement, statement of retained earnings, and balance sheet of Electronic Arts, Inc., are listed below in alphabetical order. Solve for the missing amounts, and explain...
-
ABC is a relatively newer product costing method that assigns overhead costs to a cost object on a cause and effect basis. By contrast, traditional costing methods rely on volume-based allocation...
-
Sally's Silk Screening produces specialty T-shirts that are primarily sold at special events. She is trying to decide how many toproduce for an upcoming event. During the event itself, which lasts...
-
Benzene and hexane are both liquids at room temperature. Do you expect benzene and hexane to be miscible? Do you expect benzene and water to be miscible? Explain. Hexane 0 Benzene
-
Because of two hydrogen bonds, carboxylic acids show a very strong attractive force between two molecules that persists even in the gas phase. Show this hydrogen bonding between two carboxylic acid...
-
Wal-Mart designs its networks so that a DC supports several large retail stores. Explain how the company can use such a network to reduce transportation costs while replenishing inventories...
-
A carload of Hg-ore containing grains of cinnabar (86%Hg by mass; density = 8.19 g/cm3) and grains of basalt (containing no Hg; density=2.84 g/cm3) is to be sampled and analyzed for mercury. The...
-
CMS reviews acute IPPS and long-term care hospital (LTCH) records for payment purposes. Documentation and coding assignment must be accurate and specific. CMS contracts with Medicare Administrative...
-
Problem 2. x3+2x+1 f(x) = = 5-x 8H xx (4 points) Without graphing the function, find the limits lim f(x) and lim f(x) analyt- ically and show your work. Specify if the limits are - or +. (1 point)...
-
For change management, answer the following questions in detail, citing some industry examples: 1. What would you do if your manager requested you change your way of working on a project? 2. What do...
-
1.Sony has just released a new CD recording (okay, not new because we don't buy CDS) but anyway.Here is some cost and price information: CD Disc and Packaging (material and labor) $1.75/CD...
-
Describe a situation where you have used a self-generated reward. Was it effective?
-
On March 31, 2018, Gardner Corporation received authorization to issue $30,000 of 9 percent, 30-year bonds payable. The bonds pay interest on March 31 and September 30. The entire issue was dated...
-
Evaluate *x sin t t X lim x-3 x - 33 - dt
-
How would you synthesize racemic disparlure (Problem 18.46) from corn- pounds having ten or fewer carbons?
-
Treatment of 1, 1-diphenyl- 1, 2-epoxyethane with aqueous acid yields diphenylacetaldehyde as the major product. Propose a mechanism for the reaction. Ph H30+ PHCHCH Ph Ph
-
How would you prepare o-hydroxyphenyl-acetaldehyde from phenol? More than one step is required. HO o-Hydroxyphenylacetaldehyde CH-CO
-
Due to the relationship of financial statements, the statement of stockholders' equity links the income statement to the balance sheet. True or False?
-
Troy Engines, Limited, manufactures a variety of engines for use in heavy equipment. The company has always produced all of the necessary parts for its engines, including all of the carburetors. An...
-
Trey is single and has no qualifying child. His adjusted gross income is $12,355. In order to claim the Earned Income Tax Credit, he must meet which of the following requirements? He cannot be the...

Study smarter with the SolutionInn App


