Give the product(s) that would be expected on reaction of 3-pentanone with 1 equivalent of LDA, followed
Question:
Give the product(s) that would be expected on reaction of 3-pentanone with 1 equivalent of LDA, followed by addition of 1 equivalent of
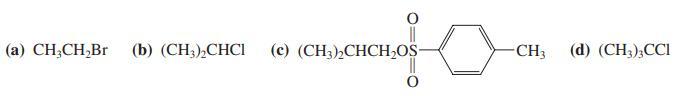
Transcribed Image Text:
(а) CH,СH,Br (b) (CH),СНCI (с) (CH)-СНCH,OS- CH3 (d) (CH),СCI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
Alkylation of Ketones Ketones can be alkylated by forming their enolate using a strong base such as ...View the full answer

Answered By

Ludhovik Luiz Madrid
I've helped students in their academics. My objective is to engage in teaching roles that aims for appreciation, critical thinking, and creativity in STEM education through inclusive and innovative methods. Currently I am a graduate student in UP Diliman, studying polymer chemistry.
In UP ALCHEMES, as a member of the Academic Affairs Committee, I spearheaded reviews and tutorials on college mathematics, general chemistry, elementary physics, and chemical engineering courses. I also administered reviewers and sample exams to students. In Diliman Learning Resource Center, I was a Chemistry Tutor Head. I spearheaded free reviews and sample exams for general chemistry courses. I also provide home-based tutorials for students in Philippine Science High School - Main Campus (premiere STEM high school in the country) in their math and science subjects.
I have the initiative to help understand the strengths and weaknesses of my tutees, and I always encourage them to push their limits.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
a. Give the product(s) that would be obtained from the reaction of cis-2-butene and trans-2 butene with each of the following reagents. If the products can exist as stereoisomers, show which...
-
Give the product(s) of each of the following reactions: a. Benzoic acid + HNO3/H2SO4 b. Isopropylbenzene + cyclohexene + HF c. Naphthalene + acetyl chloride + AlCl3 followed by H2O d. o-methylaniline...
-
Give the product(s) obtained from the reaction of each of the following compounds with Br2/FeBr3: a. b. c. d. O-C COCH3 NO2 CH3O
-
Rio Tinto is a listed company in the mining and metals production industry. Some information drawn from its 2020 annual report is shown below. Safety and health performance 2020 2019 2018 Fatalities...
-
Plaintiffs in this case are the heirs and assignees of three Marx brothers, Groucho, Chico, and Harpo. They brought this action alleging that defendants appropriated the right of publicity in their...
-
Showing the details, calculate the following expressions or give reason why they are not defined, when Au, u T A
-
1 Why do you think so few others have adopted the same approach? Visit these websites (or others of similar companies of which you learn): www.oticon.com www.gore.com www.apple.com www.bmw.com...
-
Compare and contrast the Lewin/Schein model with the Kotter framework. What is the same and what is different?
-
10. Discuss the effect, if any, each of the following should have on the recognition of future events: a. The probabilistic nature of future events b. Management intent c. Conservatism d. Future...
-
Dwight Donovan, the president of Rundle Enterprises, is considering two investment opportunities. Because of limited resources, he will be able to invest in only one of them. Project A is to purchase...
-
Propose a mechanism for the following reaction. (Take note of all of the products that are formed and base your answer on the mechanism for acid-catalyzed bromination of acetone shown below) CI...
-
Give the product(s) of the following reaction sequences. 1. , * 2. 1. , * 2. (CH),CH.CI 3. ', . CH,Br 3. H., . () CH,CO (b) CH2CHO
-
Could a depth interview be conducted via the Internet? What are the advantages and disadvantages of this procedure over conventional depth interviews?
-
(1) A test balloon has an accelerometer attached to it. After you release it and start collecting data it is 5 ft in front of you and 16 ft above you, and it is moving 5 ft/s to your left and 4 ft/s...
-
484 ... Age of Accounts as of June 30, 2019 1-30 31-60 61-90 Over 90 Customer Name Days Days Days Days Total Balance Canyon Youth Club $ 250 $ 250 Crazy Tees 200 $ 150 350 Early Start Daycare $500...
-
CLT HW Score: 0/19 0/19 answered Question 4 < = 31. You intend to draw a A population of values has a normal distribution with = 232.9 and random sample of size n = 165. Please show your answers as...
-
Q-3: Estimate fxy dx + x2 dy: where c is given by [Hint: Use Green's theorem -1
-
1. Determine completely the resultant of the four forces shown in the figure. Each force makes a 15 angle with the vertical, except the 200 N force which is vertical. Find the action line (position)...
-
Trinkle Company made several purchases of long-term assets during the year. The details of each purchase are presented here. New Office Equipment 1. List price: $60,000; terms: 2/10, n/30; paid...
-
Provide a few individual examples who revealed what aspects of emotional intelligence?
-
Express the root mean square deviation {(M2) - (M} 2} 1/2 of the molar mass of a condensation polymer in terms of p, and deduce its time dependence.
-
Calculate the average polymer length in a polymer produced by a chain mechanism in which termination occurs by a disproportionate reaction of the form M +M M + M.
-
Autocatalysis is the catalysis of a reaction by the products. For example, for a reaction A -> P it may be found that the rate law is v = k[A] [P] and the reaction rate is proportional to the...
-
On March 1, 2018, Stratford Lighting issued 10% bonds, dated March 1, with a face amount of $690,000. The bonds sold for $678,000 and mature on February 28, 2038 (20 years). Interest is paid...
-
Which of the following activities would result in an decrease in cash from financing activities for a firm? i. Selling equipment for cash ii. Issuing stock iii. Buying back treasury shares iv....
-
The following transactions occurred during 2020. Assume that depreciation of 10% per year is charged on all machinery and 5% per year on buildings, on a straight-line basis, with no estimated salvage...

Study smarter with the SolutionInn App


