Give the two theoretically possible Baeyer-Villiger products from each of the following compounds. Indicate which one is
Question:
Give the two theoretically possible Baeyer-Villiger products from each of the following compounds. Indicate which one is formed preferentially.
(a)
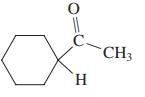
(b)
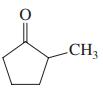
(c)
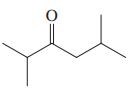
(d)
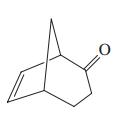
(e)
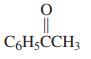
Transcribed Image Text:
C. CH3 H.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
BaeyerVilliger is the oxidation of ketone to give ester in presence of peracid and th...View the full answer

Answered By

Prajoy Kumar Mitra
Well being honest I want to say that Chemistry is my first priority. I can help you in every possible way in the field of Chemistry. I believe knowledge needs to be shared. And regarding experience I would like to say that, recently I have completed my Masters in Chemistry & also Qualified the GATE exam. Though I don't have the teaching experience but I do have good teaching skills along with sufficient knowledge. And I'm confident enough to give you a better concept in Chemistry along with certain entrance preparations.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Give two names for each of the following compounds: a. b. c. d. e. f. CHCH2CHH CH CH3 CH,CH,CH CCH,CH,CH CH3CHCH2CCH2CH2CH3 CH3 CH CH2CH2CH CH3CH2CHCH2CH2CH lia CH-CHCCH-CH-CH-CH3
-
Give the systematic name for each of the following compounds: a. b. c. d. e. CH CH,CHCH,CH CHCH NHCH3 CH3 CH3 CH CH-CHCH2CHCH CH3 HCH3 H2CH3 CH CHCHCH,CH CH CI CH CH2CH2CH2CHCH2CH2CH2CHa CH,CCH2CH3...
-
Give IUPAC names for the following compounds. (a) (b) (c) (d) (e) (f) Ph CH3C C CH CH H3C CH3 - (CH3)3C C-CH (CH3)CH2CH3 CH CHC CC-OH CH,CH CH C-C CH
-
The receiver most commonly used in AM and FM radio broadcast is the so-called superheterodyne receiver. Answer the following questions about this receiver. a. Draw the block diagram of a...
-
Can protectionism cause trade deficits? Recall the model of U.S.-China trade presented in Chapter 6, in which the United States exports plastics to China and China exports apparel to the United...
-
What are the three types of nodes in a scene graph?
-
Presented below are the stockholders' equity sections for AMR Corporation for 2004 and 2003. All amounts are in millions, except number of shares and par value. (a) Explain why common stock is...
-
The customer billing and cash receipts functions of Robinson Company Ltd, a small paint manufacturer, are attended to by a receptionist, an accounts receivable clerk, and a cashier who also serves as...
-
Assume that the firm has $40 million of debt outstanding and $80 million of equity outstanding. The bonds are priced at par and have a coupon rate of 8%. The common stock's beta is 9. The current...
-
Caden Healey is a realtor. He organized the business as a corporation on December 16, 2017. The business received $60,000 cash from Healey and issued common stock. Consider the following facts as of...
-
Formulate a detailed mechanism for the Baeyer-Villiger oxidation of the ketone shown in the margin. %3=
-
Propose efficient syntheses of each of the following molecules, beginning with the indicated starting materials. (a) (b) (c) from H;C HO, H
-
The 8-ft-wide portion ABCD of an inclined, cantilevered walkway is partially supported by members EF and GH. Knowing that the compressive force exerted by member EF on the walkway at F is 5400 lb,...
-
For all the benefits they bring to business, social media and other communication technologies have created a major new challenge: responding to online rumors and attacks on a company's reputation....
-
Directions: Put your feet in the shoes of the business owner and suggest specific ways on how a business can gain profit and how it can be avoid loss Ways to Gain Profit 1. 2. 3. 4. 5. 1. 2. 3. 4. 5....
-
Define business intelligence Briefly discuss how your organisation can use business intelligence to improve decision-making Please see below rubric as guidance. Kindly list references in APA 7th...
-
Mr Santos apply for college educational plan for his 3 children .The 3 children ages are 6 yrs old , 3 yrs old and 1 yr old. The fund will be set-up the deposit of a fixed sum on the child's current...
-
Recovery Centers of America needs to acquire new vehicles that will cost $2.5 million across its six state service area. It plans to use the vehicles for three years, at which time new vehicles will...
-
Repeat the previous exercise using Java Lock variables. Data from Repeat the previous exercise Give amore efficient solution to the previous exercise that avoids the use of notify All. (Warning: It...
-
What mass of H2 will be produced when 122 g of Zn are reacted? Zn(s) + 2HCl(aq) ( ZnCl2(aq) + H2(g)
-
Determine the degree of un-saturation for these compounds.
-
Draw a 3s atomic orbital and compare it to a 2s orbital.
-
Show an atomic orbital energy level diagram for these atoms: (a) Si (b) Al (c) Cl
-
The price of Time Squared Corp. stock will be either $77 or $93 at the end of the year. Call options are available with one year to expiration. T-bills currently yield 6 percent. The current price of...
-
LBJ Enterprises is issuing new bonds for a capital budgeting project. The bonds will have 21.00 year maturities with a coupon rate of 7.34% APR with semi-annual coupon payments (assume a face value...
-
Procter and Gamble (PG) paid an annual dividend of $ 1.65 in 2009. You expect PG to increase its dividends by 7.4 % per year for the next five years (through 2014), and thereafter by 3.2 % per year....

Study smarter with the SolutionInn App


